Application of bufadienolide compounds having amino acid chains in preparing anti-tumor drugs
A technology of bufadienolide and anticancer drug, which is applied in the application field of bufadienolide compound with amino acid chain in the preparation of anticancer drug, and can solve the problems of unreported anti-colon cancer and gastric cancer activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: preparation process of toad skin fraction
[0034] The skin of the giant toad is collected from Linyi, Shandong, and it has been identified as authentic by Yang Xiaoping, Xiyuan Chinese Medicine Research Office, China Academy of Chinese Medical Sciences.
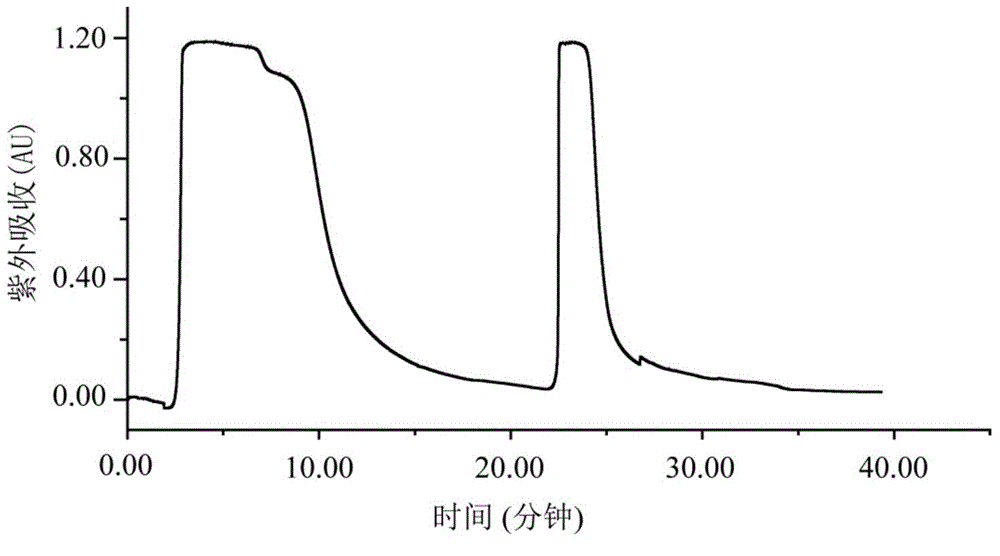
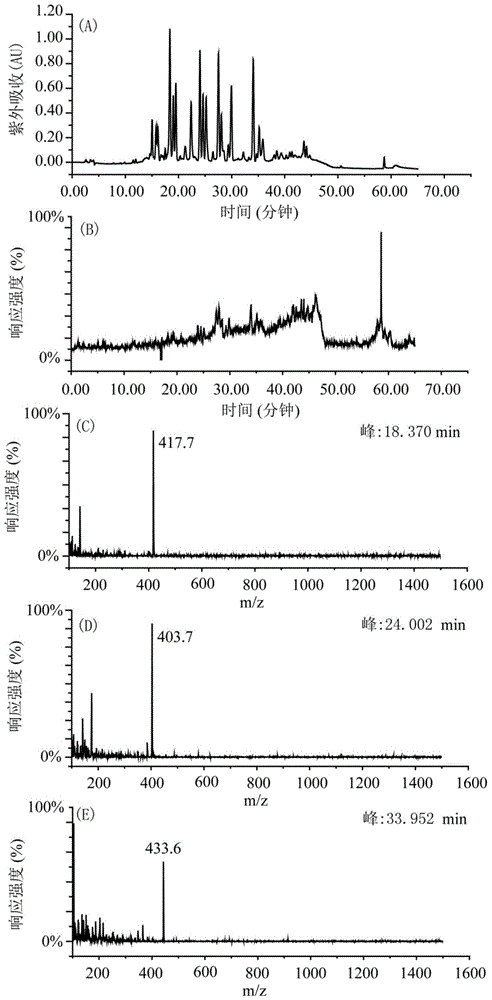
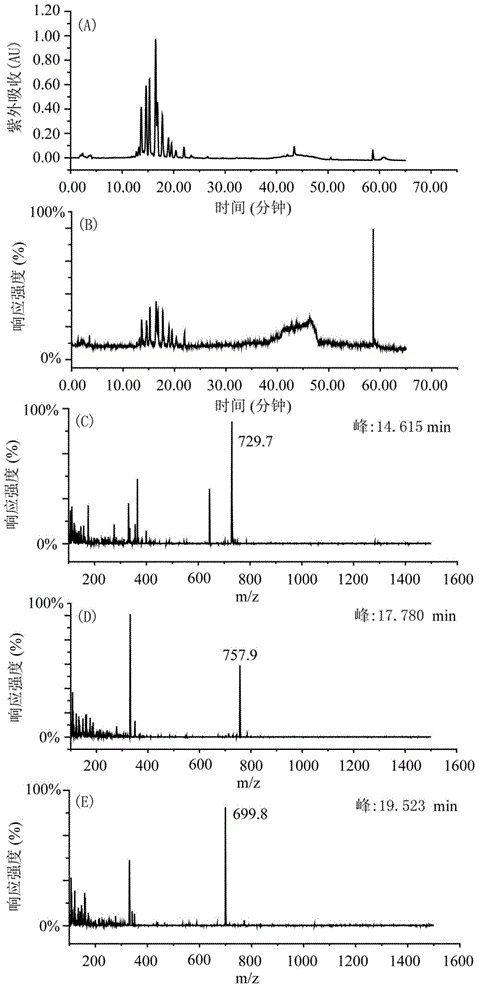
[0035] Weigh 200g of shredded toad skin, add 1.4L of pure methanol according to the material-liquid ratio of 1:7, soak for two days, reflux at 80°C for 4 hours, filter to obtain the filtrate, add 1.4L of pure methanol to the filter residue, and boil slightly at 80°C Reflux for 4 hours, filter to obtain the filtrate, and extract twice in total. The two extracts were combined to obtain 2730 mL, concentrated under reduced pressure at 60° C. and evaporated to dryness to obtain 7 g of the total extract. Dissolve the total extract with 160mL 65-75% methanol-water (V / V), extract with 160mL n-heptane, extract the methanol part with 160mL n-heptane again, extract twice, and then depressurize the methanol part at ...
Embodiment 2
[0037] Example 2: Establishment of 3D cell spheroid screening model
[0038] Human colon cancer cells HT-29 and human gastric cancer cells HGC-27 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; ULA-96 well plates were purchased from Corning; toad skin fractions were fractions 1-11 obtained in Example 1; inverted microscopes were purchased from OLYMPUS.
[0039] HT-29 cells and HGC-27 cells in the logarithmic growth phase were seeded in ULA-96 well plates, so that the number of cells in each well plate were 20K, 10K, 5K, 2.5K, 1.25K and 0.625K, The inoculation volume is 200 μL / well, the medium used for HT-29 cells is McCOY's 5A containing 10% fetal bovine serum, the medium used for HGC-27 cells is RPMI-1640 containing 20% fetal bovine serum, and each cell density is repeated three times , using an inverted microscope to record micrographs of 3D cell spheroid morphology every 24 hours for 4 consecutive days. The result is as Figure 5 and Im...
Embodiment 3
[0040] Example 3: Research on the Effect of Amino Acid Chains on the Anticancer Activity of Toad Skin Compounds
[0041] Inoculate HT-29 and HGC-27 cells at the optimal density (1.25K / well) on ULA-96 well plates and culture them for 4 days. The medium used by HT-29 cells is McCOY's 5A containing 10% fetal calf serum, HGC The medium used for -27 cells is RPMI-1640 containing 20% fetal bovine serum. After growing to 3D cell spheres with optimal particle size, 100 μL of fresh medium was replaced in each well, and the cells eluted by Click XIon column were added respectively. Bufadienolactone fractions with amino acid chains and bufadienolactone fractions without amino acid chains, the final concentration of each fraction is 10 μM and 1 μM (the average molecular weight of the compound in the fraction is calculated as 500Da), each Fraction samples were repeated three times. The size of the 3D cell spheroids was recorded using an inverted microscope every 24 hours for 6 consecuti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com