Heparin-modified adriamycin liposome preparation and preparation method thereof
A technology of liposome preparation and doxorubicin lipid, which is applied in the field of pharmaceutical preparations, can solve the problems of clinical application limitations of toxic side effects, dose-dependent cardiotoxicity, and damage to myocardial cells, so as to improve bioavailability, prolong half-life in vivo, The effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
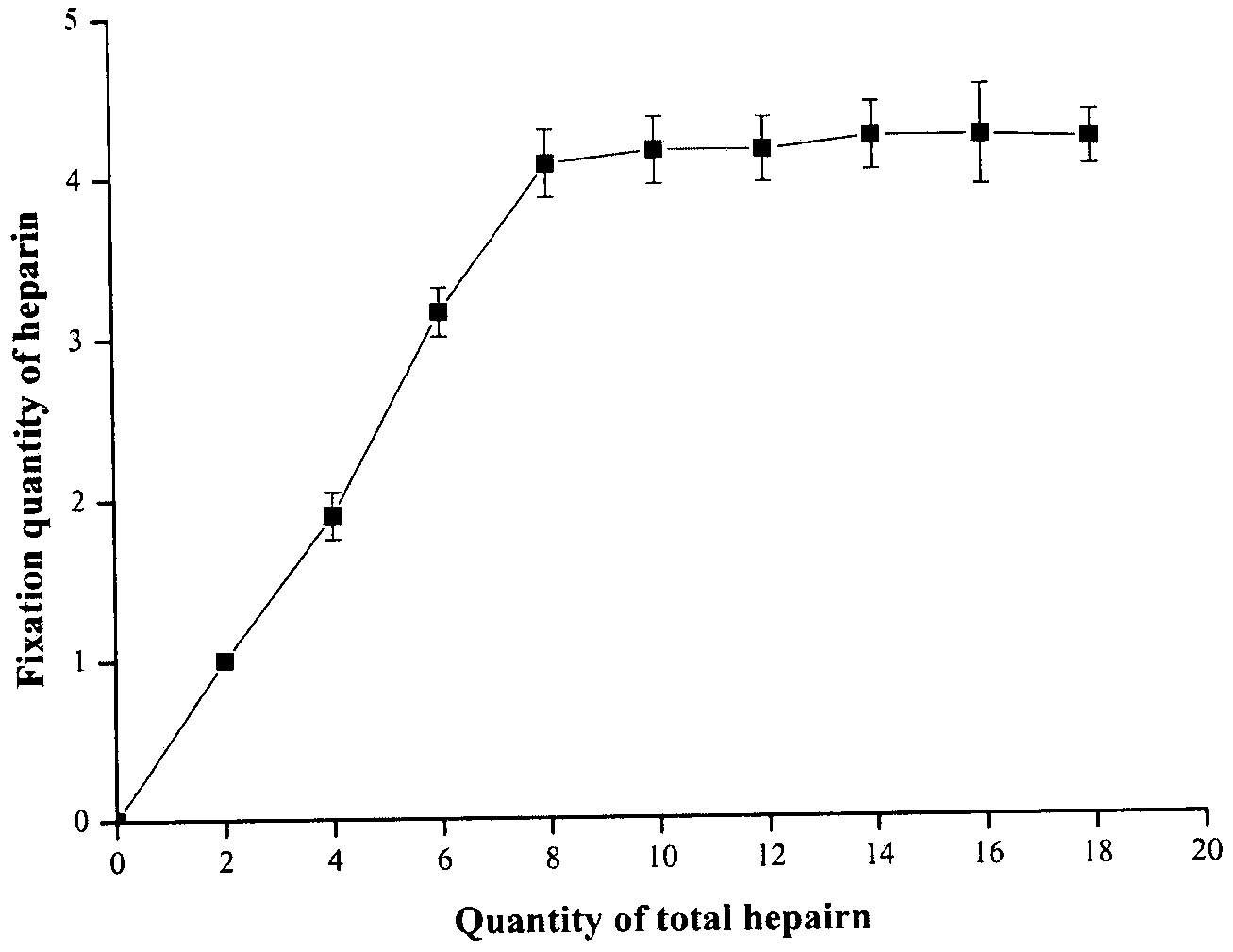
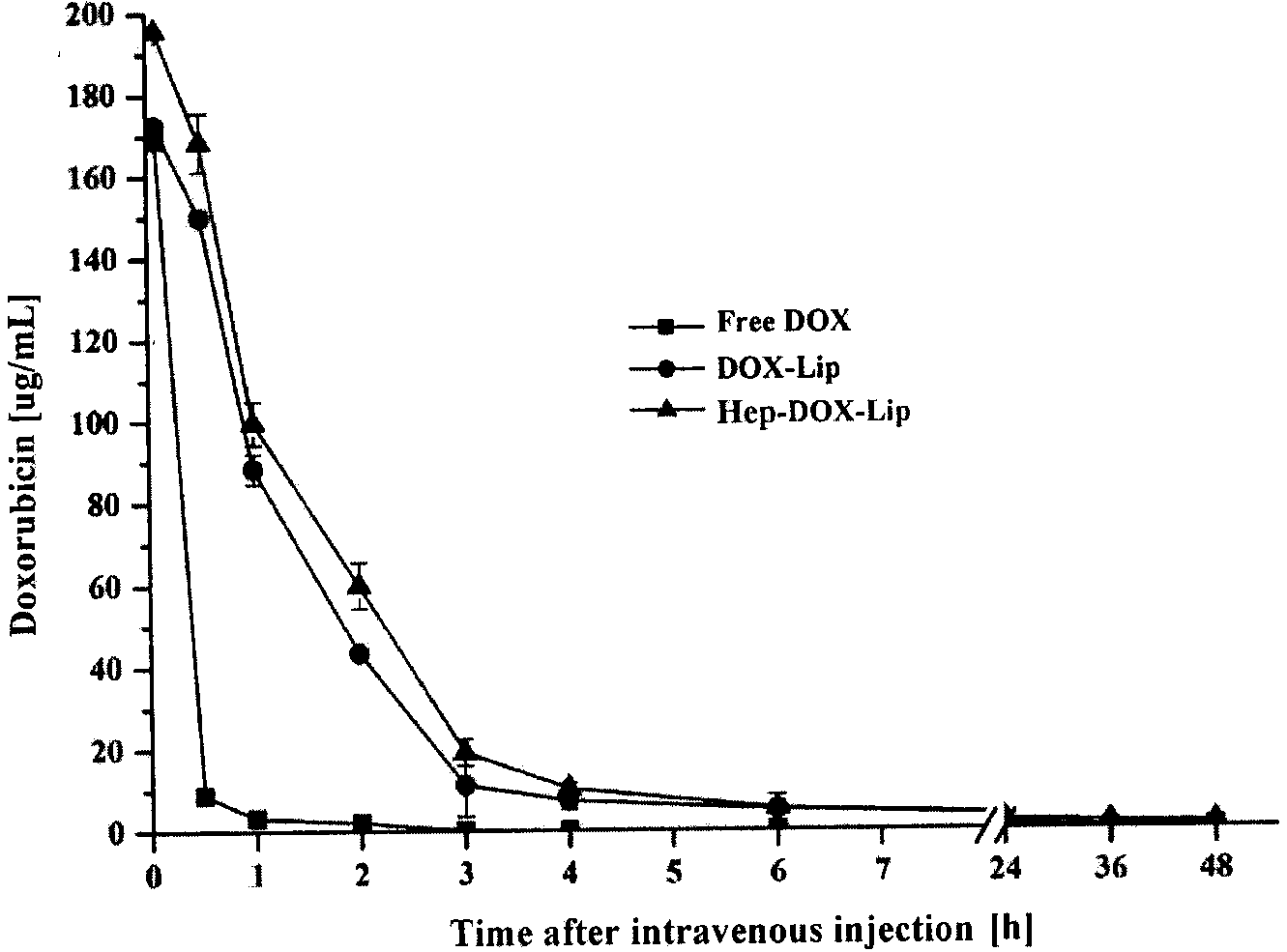
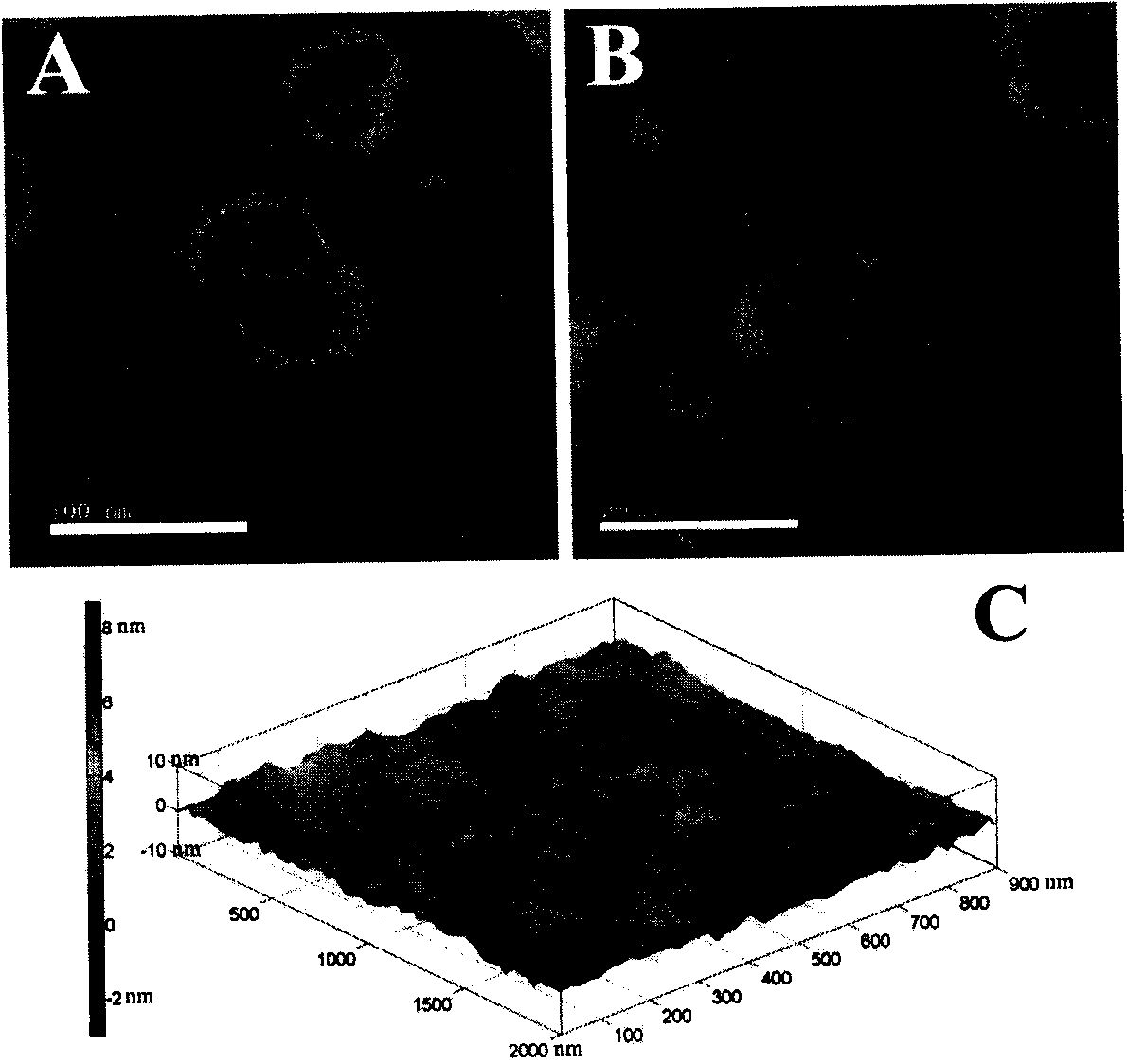
[0058] Weigh 10 mg of soybean lecithin, 1 mg of cholesterol, and 0.5 mg of dioctadecyldimethylammonium bromide respectively in prescription quantities and place them in an eggplant-shaped bottle, add an appropriate amount of dichloromethane, and ultrasonically dissolve them completely; rotary vacuum evaporation The organic solvent was removed to form a dry and uniform lipid film on the bottle wall. Add 1 mL of 300 mmol / L ammonium sulfate solution to wash the membrane, and hydrate at 37°C for 1 hour to form a liposome primary emulsion. Probe ultrasound (200W, 100 times) to obtain blank liposomes. The above blank liposomes were dialyzed in 10% sucrose solution for 3 hours (change the dialysate every 1 hour), then add adriamycin solution (1 mg / mL), and incubate in a water bath at 60°C for 30 minutes to obtain DOX-Lip. Take the DOX-Lip solution and incubate with an equal volume of enoxaparin solution (4mg / mL) for 30min at 25°C. Remove free heparin by ultrafiltration to obtain He...
Embodiment 2
[0065] Weigh 15 mg of soybean lecithin, 2 mg of cholesterol, and 0.5 mg of stearylamine in prescription quantities and place them in an eggplant-shaped bottle, add an appropriate amount of chloroform, and ultrasonically dissolve them completely; rotary vacuum evaporation removes the organic solvent, and forms a dry Uniform lipid film. Add 1 mL of 300 mmol / L ammonium sulfate solution to wash the membrane, and hydrate at 37°C for 1 hour to form a liposome primary emulsion. Probe ultrasound (200W, 100 times) to obtain blank liposomes. The above blank liposomes were dialyzed in 10% glucose solution for 3 hours (change the dialysate every 1 hour), then add adriamycin solution (1 mg / mL), and incubate in a water bath at 60°C for 30 minutes to obtain DOX-Lip. Take the DOX-Lip solution and incubate with an equal volume of unfractionated heparin solution (4mg / mL) for 30min at 25°C. Remove free heparin by ultrafiltration to obtain Hep-DOX-Lip suspension. Then add an appropriate amount...
Embodiment 3
[0068] Weigh 20 mg of soybean lecithin, 1.5 mg of cholesterol, and 0.5 mg of trimethylhexadecyl ammonium bromide respectively in prescription quantities and place them in an eggplant-shaped bottle, add an appropriate amount of methanol, and ultrasonically dissolve them completely; Solvent, forming a dry and uniform lipid film on the bottle wall. Add 1 mL of 300 mmol / L ammonium sulfate solution to wash the membrane, and hydrate at 37°C for 1 hour to form a liposome primary emulsion. Probe ultrasound (200W, 100 times) to obtain blank liposomes. The above blank liposomes were dialyzed in 10% lactose solution for 3 hours (change the dialysate every 1 hour), then add adriamycin solution (1 mg / mL), and incubate in a water bath at 60°C for 30 minutes to obtain DOX-Lip. Take the DOX-Lip solution and incubate with an equal volume of dalteparin sodium solution (4mg / mL) for 30min at 25°C. Remove free heparin by ultrafiltration to obtain Hep-DOX-Lip suspension. Then add an appropriate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com