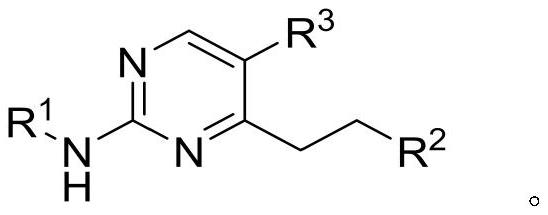
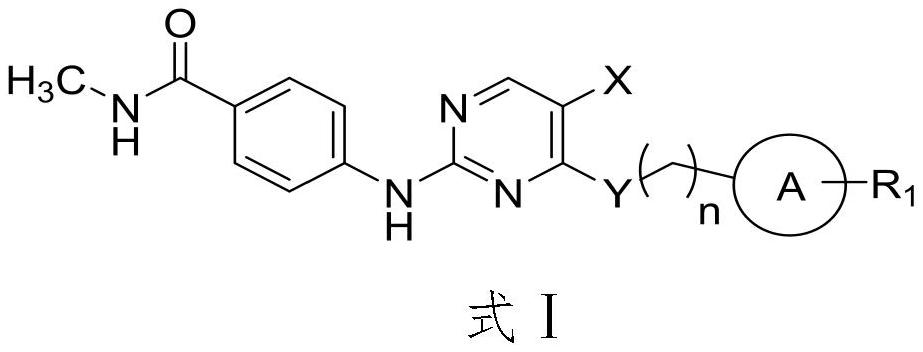
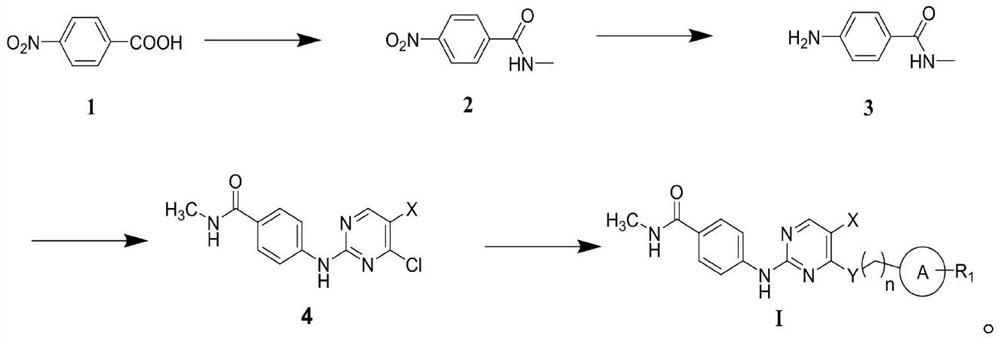
2-(4-carbamoyl)anilino-4-aminopyrimidine derivatives and their applications
A carbamoyl and aminopyrimidine technology, applied in the field of 2-anilino-4-aminopyrimidine derivatives and applications, can solve the problems of few reports in research, achieve good application prospects, high yield, and significant anti-tumor activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 2-(4-carbamoyl)anilino-4-aminopyrimidine derivatives
[0033] (1) Synthesis of intermediate 2 (N-methyl-4-nitrobenzamide):
[0034] synthetic route:
[0035]
[0036] Synthesis process: take 250mL dry reactor, add 4-nitrobenzoic acid (1g, 6mmol), then add 30mL oxalyl chloride to dissolve, stir reaction on an oil bath at 65-70°C (anhydrous operation), and wait for the solution When it became a light yellow transparent liquid, the solution was spin-dried under reduced pressure to obtain a yellow solid; the yellow solid was dissolved in dichloromethane, and triethylamine (1.8 g, 178 mmol) was slowly added in an ice bath, and then 40% methylamine aqueous solution ( 287mg, 5.2mmol), stirred at room temperature for about 30 minutes; after the reaction was complete, the solvent was spin-dried under reduced pressure, the spin-dried mixture was dissolved with ethyl acetate and an appropriate amount of water and the solution was extracted with ethyl acetate for 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com