Platinum complex taking 2-benzoylpyridine thiosemicarbazone as ligand as well as synthesis method and application of platinum complex
A technology of benzoylpyridine thiosemicarbazone and benzoylpyridine, applied in the field of platinum complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
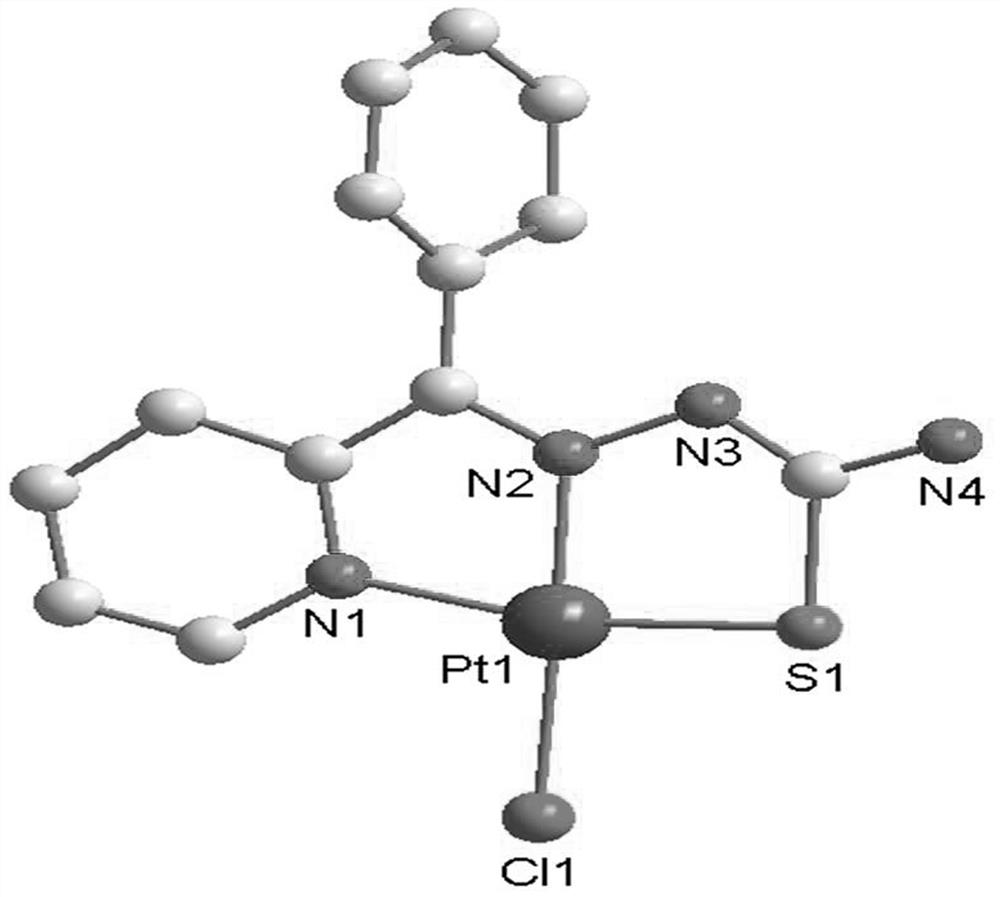
[0028] The synthesis of C1 platinum complex, the specific synthesis method is:
[0029] (1) Weigh 2-benzoylpyridine (1.83g, 10mmol) and place it in an EP tube containing 20mL of methanol, dissolve it ultrasonically, weigh thiosemicarbazide (0.91g, 10mmol) and add it to the above solution, then add 500μL of ice Acetic acid, put it in a 65°C oven after dropping, react for 1 day, the final solution color is orange and take it out; then when the temperature of the solution is not much different from room temperature, filter it into a 50mL beaker and place it in a fume hood to slowly volatilize , after a few days, light yellow crystals were precipitated, and the solution was filtered to obtain ligand L1;
[0030] Yield 73%. Anal. Calcd (%) for C13H12N4S: C, 60.91%; H, 4.72%; N, 21.86%; S, 12.51%. Found: C, 61.02%; H, 4.53%; N, 21.55% ;S,12.90%.IR(KBr,cm-1) 3303s,3134vs,3022m,1615s,1459s,1371s,1249s,955s,852s,782s,702s,699s,588s.MSI-MS:m / z=257.08for [M+H]+;
[0031] (2) Weigh the...
Embodiment 2
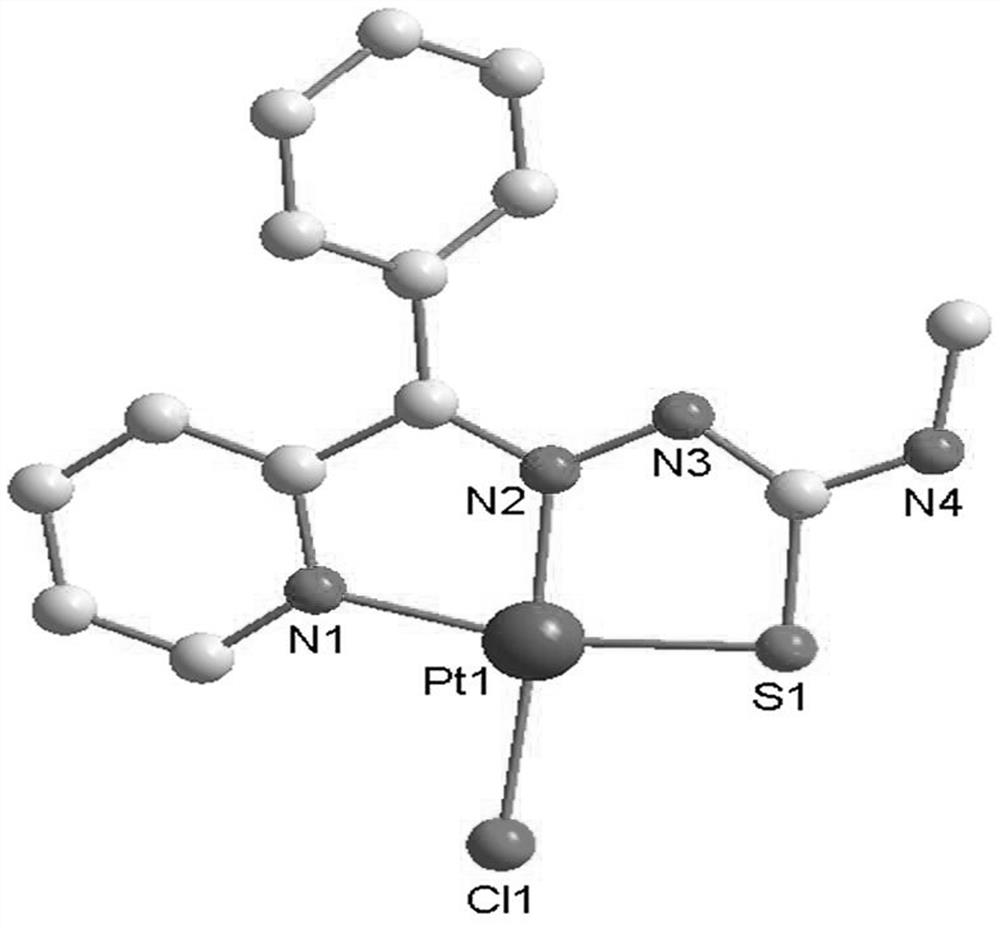
[0034] The synthesis of C2 platinum complexes, the specific synthesis method is:
[0035] (1) Weigh 2-benzoylpyridine (1.83g, 10mmol) and place it in a round-bottomed flask containing 20mL of methanol, stir to dissolve, weigh 4-methylthiosemicarbazide (1.05g, 10mmol) and add it to the above solution , then add 500 μL of glacial acetic acid, after the dropwise addition, stir and react at 65°C for 8 hours, the color of the solution is orange, take it out, filter it into a 50ml beaker, place it in a fume hood and volatilize slowly, after a few days, a light yellow crystal precipitates , filter the solution to obtain the ligand L2;
[0036] Yield 68%.Anal.Calcd(%)for C14H14N4S:C,62.20%;H,5.22%;N,20.72%;S,11.86%.Found:C,62.835%;H,5.045%;N,21.15% ;S,12.35%.IR(KBr,cm-1) 3296s,3054vs,2937m,1539s,1470s,1311s,1249s,1110s,1043s,818s,697s,649s,599s.MSI-MS:m / z=293.08for [M+Na]+;
[0037] (2) Weigh the ligand L2 (0.0135g, 0.05mmol) and Pt(DMSO) obtained in step (1) 2 Cl 2 (0.021 g, 0.0...
Embodiment 3
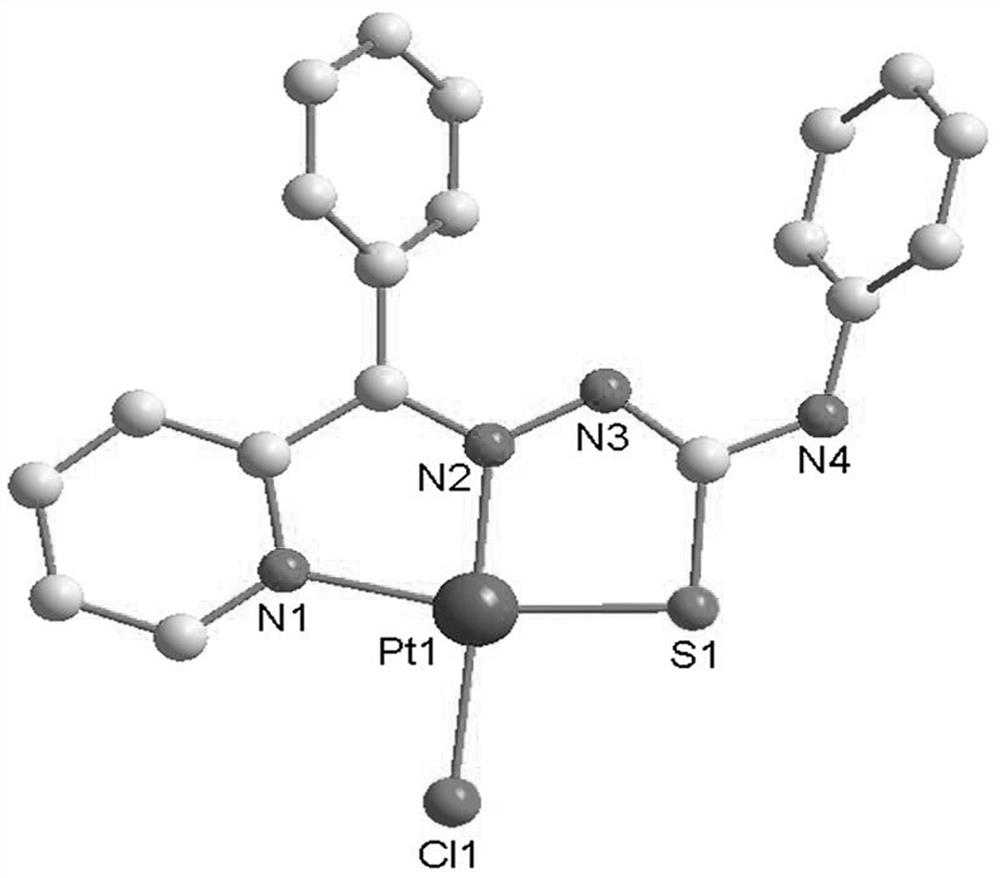
[0040] The synthesis of C3 platinum complexes, the specific synthesis method is:
[0041] (1) Weigh 2-benzoylpyridine (1.83g, 10mmol) and place it in a round-bottomed flask containing 20mL of methanol, stir to dissolve, weigh 4-phenylthiosemicarbazide (1.67g, 10mmol) and slowly add to the above Then add 500μL of glacial acetic acid, after the dropwise addition, stir and react for 8 hours at 65°C, the color of the solution is orange, take it out, and when the temperature of the solution is not much different from room temperature, filter it into a 50mL beaker and place in Slowly volatilize in the fume hood, and after a few days, light yellow crystals are precipitated, and the solution is filtered to obtain the ligand L3;
[0042] Yield 71%, Anal. Calcd (%) for C19H16N4S: C, 68.65%; H, 4.85%; N, 16.85%; S, 9.65%. Found: C, 69.32%; H, 4.77%; N, 17.10% ;S,9.97%.IR(KBr,cm-1)3299s, 3051vs,1533s,1439s,1304s,1203s,1174s,1106s,791s,756s,696s,655s,499s. MSI-MS:m / z=333.11for [M+H]+;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com