Modified arginine deiminase
A technology of arginine deiminase and arginine, applied in hydrolytic enzymes, medical preparations containing active ingredients, peptide/protein components, etc., can solve the problems of immunogenic toxicity, unstable linkers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Production of recombinant ADI
[0072] The gene of arginine deiminase is synthetic according to the sequence of Mycoplasma arginini published in Infect.Immun., 58:3788-3795 (1990) such as T.Ohno, and open reading frame comprises 1230 base pairs (Fig. 1 and SEQ ID NO: 1), wherein 5 codons TGA encoding tryptophan are unintentional codons of Escherichia coli and changed to TGG. SEQ ID NO: 1 encodes a 410 amino acid arginine deiminase (shown in SEQ ID NO: 2 and Figure 2).
[0073] The expression and renaturation in Escherichia coli were carried out according to the following method: Specifically, the synthetic ADI gene was inserted into the SpeI and BamHI sites of pET32 vector (Novagen Company), and the expression plasmid pET32-ADI was constructed. pET32-ADI was transformed into conventional Escherichia coli strain BL21, and the transformed bacteria were cultured in 500ml LB medium, and the expression was induced with 1.0mM IPTG for 4 hours. Bacteria were disrupted by ult...
Embodiment 2
[0076] ADI cross-linked with TMPEG
[0077] The ADI prepared in Example 1 and TMPEG with an average molecular weight of 5000 Da were used in this example.
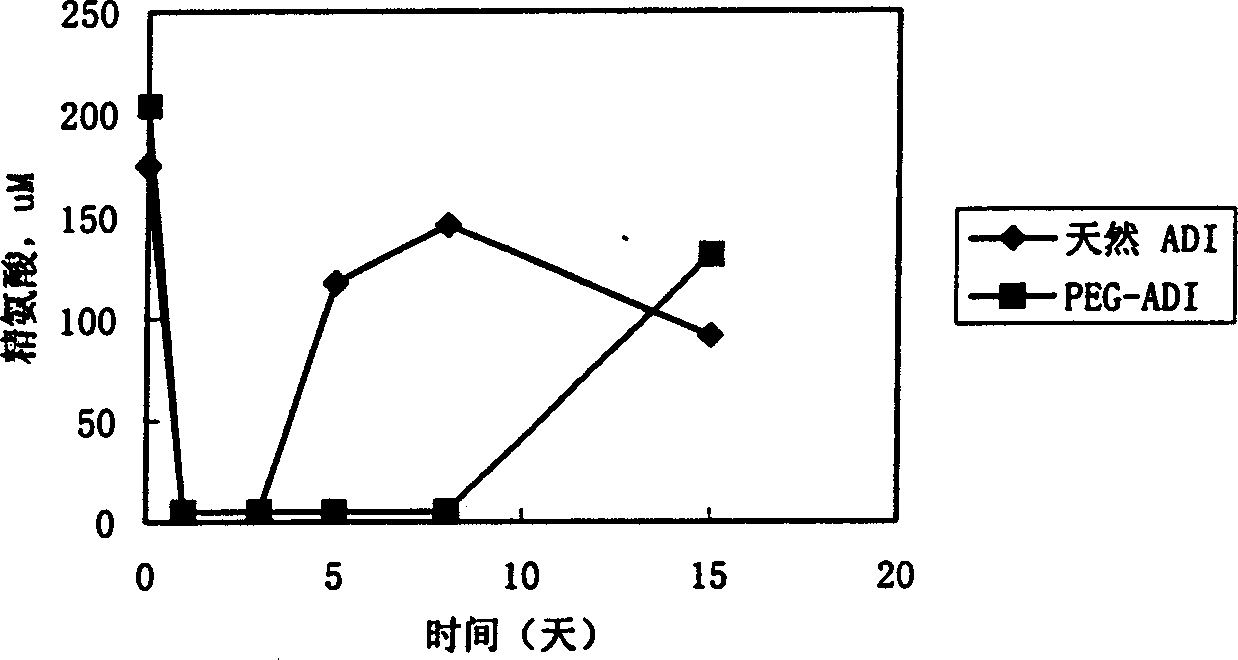
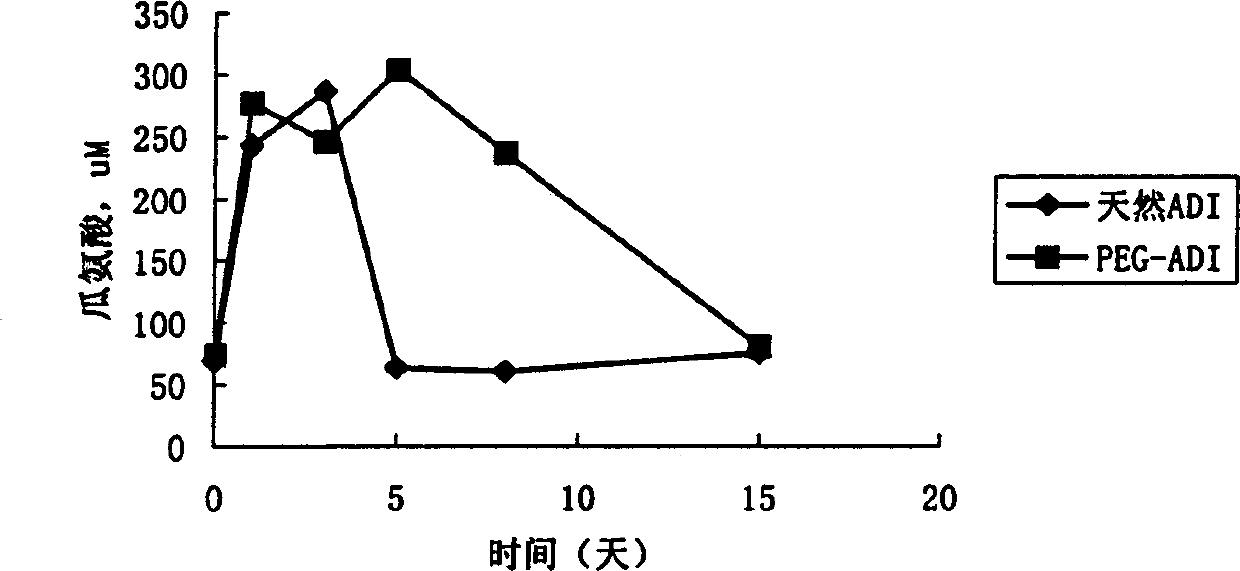
[0078] The cross-linking reaction of PEG and ADI was carried out at pH 7.5, 50 mM phosphate buffer solution with 0.125 M sodium chloride added, the mass ratio of PEG and ADI was 30:1, and stirred at room temperature for 2 hours. After the reaction, unbound PEG in the mixture was removed by ultrafiltration membrane dialysis, and the degree of modification and enzyme activity of the conjugate were measured.
[0079] The following assay was used to determine the degree of modification of arginine deiminase:
[0080] Carry out with reference to the method described in J.Biol.Chem., 252.3582-(1977) by A. Abuchowski et al., that is, titrate the free amino group with the trinitrobenzenesulfonic acid (TNBS) method, and determine the modification by comparing the change of the free amino group before and after PEG modification. d...
Embodiment 3
[0086] Cross-linking of ADI with mPEG-C1
[0087] The ADI prepared in Example 1 and mPEG-Cl with an average molecular weight of 5000 Da were used in this example.
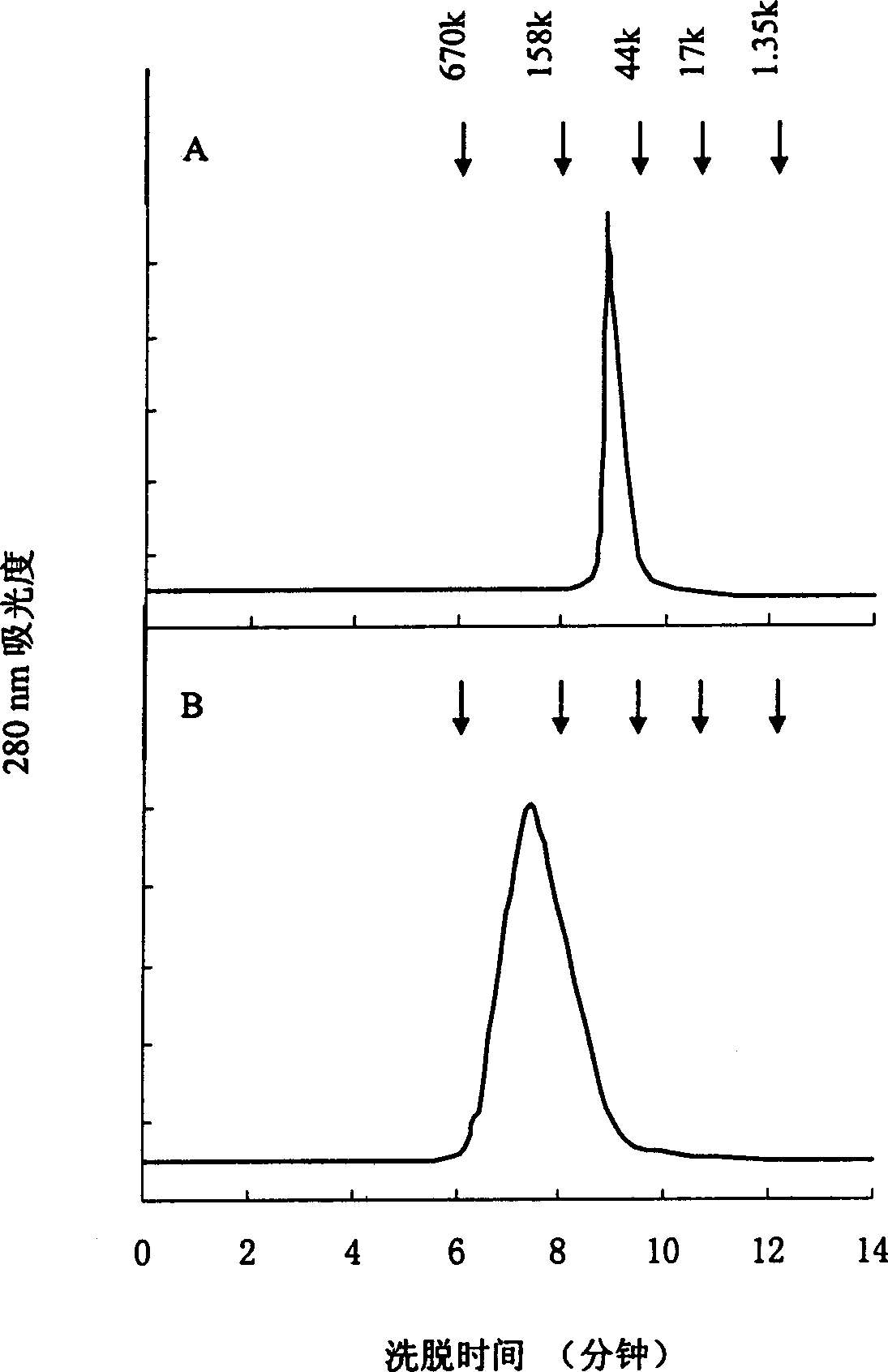
[0088] The cross-linking reaction between mPEG-Cl and ADI was carried out in 20 mM phosphate buffer at pH 7.0, the molar ratio of PEG to ADI was about 40:1, and stirred at room temperature for 2 hours. After the reaction, unbound PEG in the mixture was removed by ultrafiltration membrane dialysis, and the degree of modification and enzyme activity of the conjugate were measured. It was purified by Sephacryl S-300HR (Pharmacia) gel chromatography, and fractions with a molecular weight of about 200,000 Da were collected. In this example, after removing unbound PEG, the total modification degree of the conjugate was 53%, and the remaining enzyme activity after modification accounted for 55% of the initial enzyme activity.
[0089] The conjugate was further purified by Sephacryl S-300HR (Pharmacia) gel chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com