PEG (polyethylene glycol) modified recombinant arginine deiminase (ADI) as well as preparation method and application thereof
A technology of arginine deiminase and ADI-SC-PEG20, which is applied in the field of medical bioengineering, can solve problems such as short half-life, achieve good in vitro stability, and improve plasma elimination half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
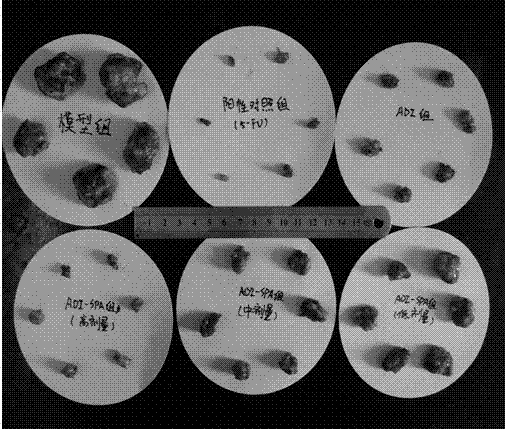
Examples
Embodiment 1
[0027] The purification of embodiment 1 ADI recombinant protein
[0028](1) Preparation of recombinant ADI crude enzyme solution: take the Escherichia coli fermentation broth expressing recombinant arginine deiminase and centrifuge to collect the bacteria, wash with 10-500mmol / L, pH 6-8 sodium phosphate buffer for 2 Second, according to the wet cell weight: buffer weight ratio of 1: 2-20, add buffer to resuspend, carry out ultrasonic crushing under ice bath conditions for 15 minutes, centrifuge the crushed liquid, and the obtained supernatant is the recombinant ADI crude enzyme liquid ; The ultrasonic crushing process is 400W, ultrasonic for 1s, stop for 3s;
[0029] (2) Purification of recombinant ADI:
[0030] Solution A: 20 mmol / L, pH 7.0 sodium phosphate buffer;
[0031] Solution B: 20 mmol / L, pH 7.0 sodium phosphate buffer, containing 1 mol / L NaCl;
[0032] Solution C: 20 mmol / L, pH 7.0 sodium phosphate buffer, containing 0.15 mol / L NaCl.
[0033] a.HiTrap TM DEAE...
Embodiment 2
[0043] Embodiment 2 PEG modification reaction
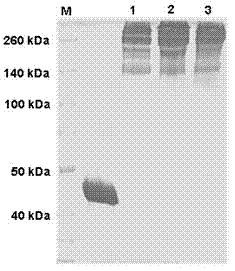
[0044] Adjust the concentration of ADI pure enzyme solution to 0.5 mg / mL and pH 8.0 with 20 mmol / L PBS buffer. According to the molar ratio of 1:120, three PEG modifiers (mPEG-SS 20 kDa , mPEG-SC 20 kDa , mPEG-SPA 20 kDa ), and stirred at room temperature for 2 h. SDS-PAGE analysis of modified products see figure 1 .
[0045] The modified product was tested for enzyme activity, modification rate, and molecular weight (SDS-PAGE). The results are shown in Table 1.
[0046] Table 1 Enzyme activity and modification rate of different ADI-PEG
[0047]
Embodiment 3
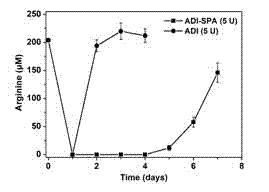
[0048] Example 3 ADI-SPA-PEG 20 PD / PK studies
[0049] ADI-SPA-PEG 20 Determination of pharmacological parameters:
[0050] (1) Experimental group settings:
[0051] ADI: intravenous injection group, intramuscular injection group, subcutaneous injection group;
[0052] ADI-SPA-PEG 20 : intravenous injection group; intramuscular injection group; subcutaneous injection group.
[0053] Three mice in each group were used as parallel experiments, and three kinds of drug doses were injected, namely 5 U / mouse, 1 U / mouse, and 0.2 U / mouse. The sampling time was 1 hour after injection, and then sampling was performed at a fixed time point every day for 7 consecutive days.
[0054] Blood collection site: tail vein.
[0055] (2) Determination of Pharmacodynamics parameters
[0056] Serum sample treatment: Add 10% sulfosalicylic acid solution to the serum sample at a volume ratio of 1:1, stir and mix well, let stand at 4°C for 6 h, centrifuge at 10,000 r / min for 10 min, and take t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com