Mutated arginine deiminase as well as preparation method and application thereof
A technology of arginine deiminase and arginine, which is applied in the field of mutant arginine deiminase and its preparation, can solve the problems of low fermentation unit and the like, and achieves simple ingredients and is conducive to separation and purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Construction of arginine deiminase (ADI) encoding gene, establishment of error-prone PCR system, establishment of ADI activity detection system, conditions for determining arginine and citrulline by HPLC
[0058] 1. Primer design
[0059] According to the full gene sequence of ADI and the characteristics of the expression vector pET-21a of Escherichia coli, the initial construction and the mutation primers before and after error-prone PCR (both are the same):
[0060] Primer ADI1F: 5'-CGCGGATCCATGAAAATGGAACAAGCATTG-3' (underlined bases are Bam HI recognition sites);
[0061] Primer ADI1R: 5'-ACGCGTCGACTTATTTTAGATTTTCTCTAAC-3' (bases underlined are Sal I recognition sites).
[0062] 2. PCR primers to amplify ADI target gene
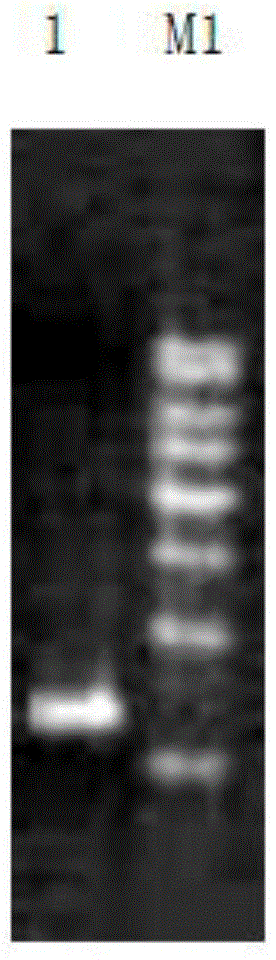
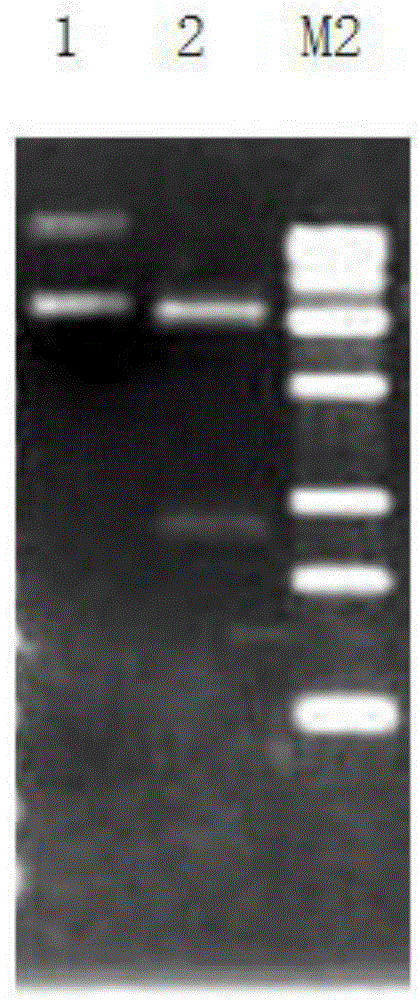
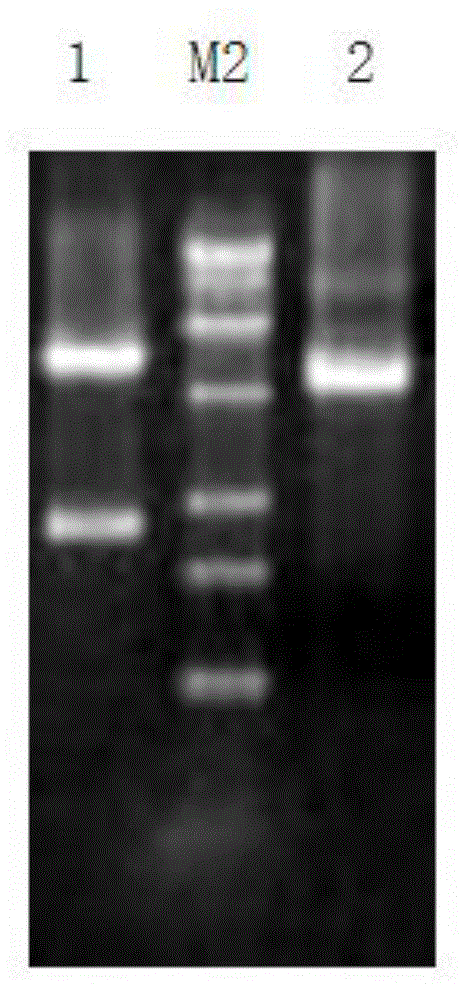
[0063] ADI1F and ADI1R were used as primers, and the plasmid PUC 57-ADI (synthesized by Nanjing KingScript Biotechnology Co., Ltd.) was used as a template to amplify the ADI gene. The polymerase used in PCR, the corresponding amplificati...
Embodiment 2
[0099] Example 2 Construction and expression of the Bacillus subtilis recombinant plasmid of the gene encoding arginine deiminase (ADI)
[0100] 1. Primer design
[0101] Cloning primers were designed according to the obtained arginine deiminase gene sequence containing five mutation sites screened in Example 1 and the multiple cloning site of pWB980 (secretion type) Bacillus subtilis.
[0102] ADI2F: CGCGGATCCCATGAAAATGGAACAAGCATTG (underlined bases are BamHI recognition sites);
[0103] ADI2R: ACGCGTCGACTTATTTTAGATTTTCTCTAAC (the underlined base is the Sal I recognition site);
[0104] 2. PCR primers to amplify ADI target gene
[0105] ADI2F and ADI2R were used as primers, and the plasmid PUC 57-ADI (synthesized by Nanjing KingScript Biotechnology Co., Ltd.) was used as a template to amplify the ADI gene. The polymerase used in PCR, the corresponding amplification buffer, and dNTP solution were all provided by TaKaRa Company purchased.
[0106] The PCR reaction system is...
Embodiment 3
[0116] Example 3 Construction and expression of recombinant plasmid of Pichia pastoris encoding gene for arginine deiminase (ADI)
[0117] 1. Primer design
[0118] According to the obtained arginine deiminase (ADI) gene sequence containing five mutation sites screened in Example 1, and the multiple cloning sites of the Pichia pastoris vector were designed to connect intracellular and extracellular expression Vector primers.
[0119] ADI3F: CCG GAATTC ATGAAAATGGAACAAGCATTG (underlined bases are EcoRI recognition sites);
[0120] ADI3R: CCG GAATTC TTATTTTAGATTTTCTCTAAC (underlined bases are EcoRI recognition sites);
[0121] 2. Construction of Pichia pastoris recombinant expression vectors pAO815-ADI and pPic9k-ADI
[0122] ADI3F and ADI3R were used as primers, and the plasmid PUC 57-ADI (synthesized by Nanjing KingScript Biotechnology Co., Ltd.) was used as a template to amplify the ADI gene. The polymerase used in PCR, the corresponding amplification buffer, and dNTP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com