Low temperature alkaline pectinase mutant with improved specific activity and thermal stability
A technology of thermal stability and mutant, applied in the field of bioengineering, can solve problems such as poor thermal stability and unfavorable industrial application, and achieve the effects of improving thermal stability, activity, specific enzyme activity and thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, Obtaining of Low Temperature Alkaline Pectin Lyase Mutant E184D / K185S
[0026] According to the nucleotide sequence of the wild-type low-temperature alkaline pectin lyase gene pel1, the point mutation primer pair is designed as follows:
[0027] E184D / K185S-F: 5'CAGC GATAGCG ACACCCTCAAT 3'
[0028] E184D / K185S-R: 5'GTGTC GCTATC GCTGTAACCATTGA 3'
[0029] Using the plasmid pET26a-pel 1 as a template, PCR amplification was performed with the designed primer pair.
[0030] PCR reaction system:
[0031]
[0032] PCR reaction conditions: pre-denaturation at 98°C for 30s, then denaturation at 98°C for 10s, annealing at 55°C for 5s, extension at 72°C for 30s, 25 cycles, and finally extension at 72°C for 30s.
[0033]The yield and specificity of the PCR products were detected by 0.7% agarose gel electrophoresis, and purified with a DNA purification kit. The purified PCR product was demethylated with DpnI, transformed into Escherichia coli XL-Gold clone com...
Embodiment 2
[0034] Embodiment 2, expression and purification of low temperature alkaline pectin lyase
[0035] Plasmids pET26b-pel1 and pET26b-pelE184D / K185S were respectively transferred into BL21(DE3) to obtain recombinant bacteria BL21(DE3) / pET26b-pel1 and BL21(DE3) / pET26b-pelE184D / K185S.
[0036] The two recombinant bacteria BL21(DE3) / pET26b-pel1 and BL21(DE3) / pET26b-pelE184D / K185S were respectively cultured in TB medium containing 50 μg / ml kanamycin, and cultured at 37°C for 3 hours; OD 600 When =2.0-2.5, add IPTG to its final concentration in LB medium of 0.5mM, transfer to 18°C and continue culturing for 25-30h.
[0037] Collect the fermentation supernatant by centrifugation at 5000rpm and 10min, desalt the supernatant with a desalting column GE HiTrap Desalting, and elute with solution C (50mM Tris-HCl, pH 7.0), and then pass the eluate through an anion exchange column GEHiTrap SP FF, first use solution D (20mM Tris-HCl, pH7.5) to elute the impurity protein, then use solution E...
Embodiment 3
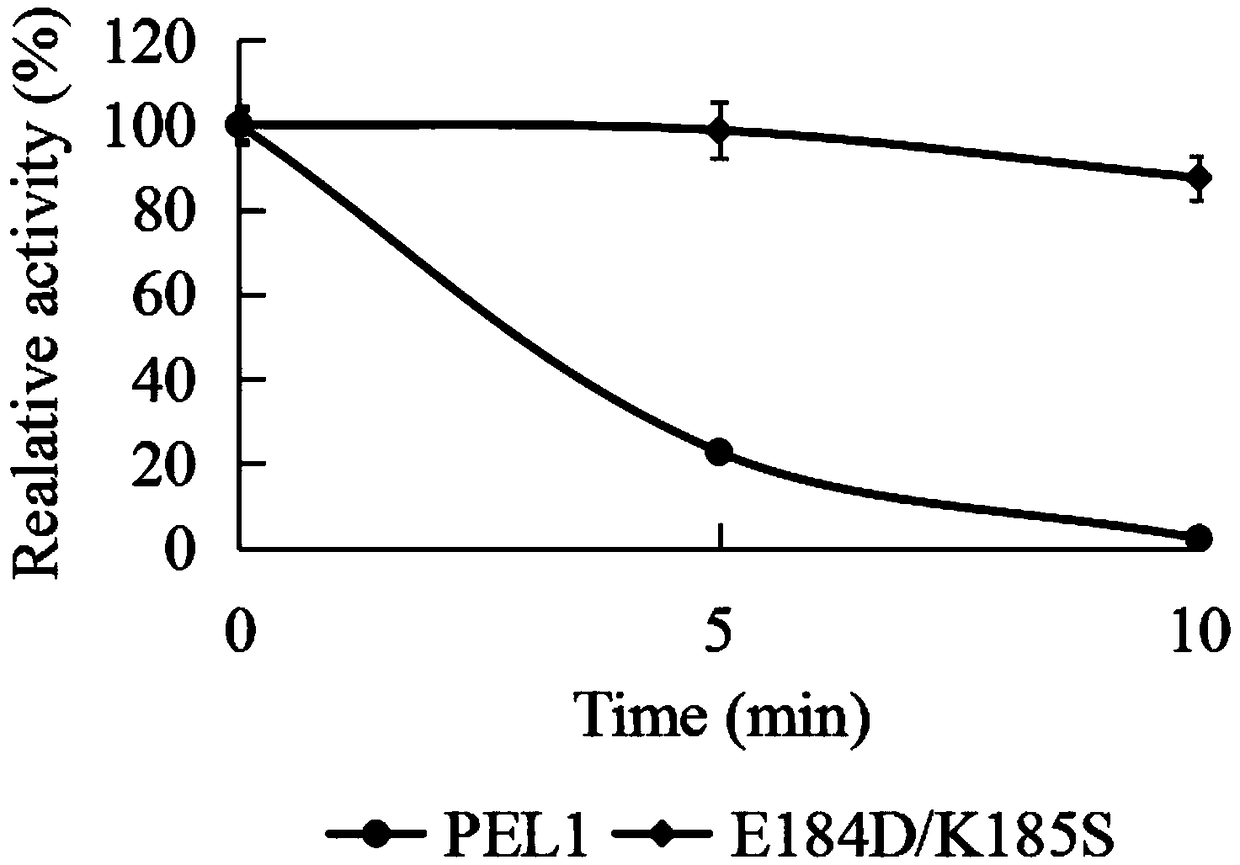
[0039] Embodiment 3, the comparison of the specific enzyme activity of low temperature alkaline pectin lyase Pel1 and E184D / K185S
[0040] 1. Determination of pectinase activity
[0041] Preheat the PGA solution at 30°C for 5 minutes; take 20 μl of enzyme diluent, add 2ml of buffer solution containing 0.2% PGA to start the enzymatic reaction; the reaction conditions are 30°C, 15min, and then stop the reaction with 3ml of 0.03mol / L phosphoric acid. The absorbance value was measured at 235nm.
[0042] Blank control: inactive enzyme solution reaction (namely: first take 3ml of phosphoric acid and mix with the enzyme solution to be tested, react according to the above reaction conditions, and then add the substrate to terminate the reaction).
[0043] Enzyme activity calculation:
[0044]
[0045] Where: 4600(L . mol -1 cm -1 )—the molar absorptivity of unsaturated PGA at 235nm;
[0046] t (min)—enzymatic reaction time (in the linear range of enzyme reaction);
[0047] b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com