Higher-substrate-specificity keratinase mutant and preparation method thereof
A technology of keratinase mutation and keratinase, which is applied in the fields of botanical equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc. Problems such as poor thermal stability of protease, to achieve the effect of improving specificity, enhancing specificity, and improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
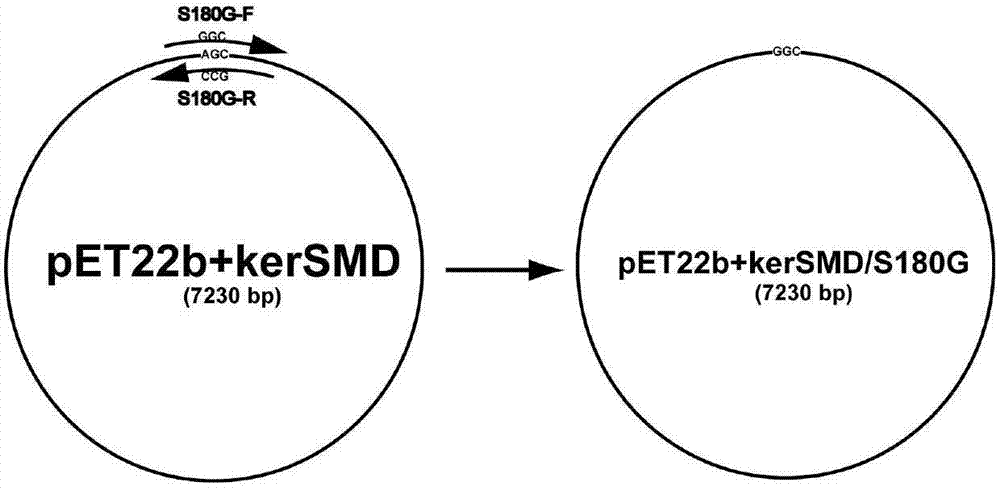
[0026] Example 1 Construction of the gene encoding keratinase mutant S180G
[0027] (1) First use a pair of 30bp complementary primers S180G-F and S180G-R to contain Stenotrophomonas maltophilia BBE11-1 (preserved in China Center for Type Culture Collection on April 3, 2011 , the preservation number is CCTCC No: M 2011193) the plasmid pET22b+kerSMD of the keratinase gene kerSMD is used as a template, and the rolling circle PCR amplification is carried out as follows: figure 1 . The reaction conditions are: pre-denaturation at 95°C for 5 minutes, followed by a cycle: denaturation at 98°C for 10 seconds, annealing at 55°C for 10 seconds, extension at 72°C for 7 minutes and 50 seconds, 15 cycles; extension at 72°C for 10 minutes, and then cooling down to 12°C to obtain the final reaction solution. The DNA amplification enzyme used was Primer STAR from TaKaRa Company, and the formula was used according to the product manual.
[0028] (2) Treat the amplified PCR product with DpnI...
Embodiment 2
[0033] Embodiment 2 cultivates recombinant bacterium fermentation production keratinase
[0034] Transform E.coli BL21 into E.coli BL21 to obtain the genetically engineered bacterium expressing keratinase with the recombinant expression vectors carrying the coding mutants E208S, Y215G, Y215S, Y215A, S180G / Y215A and S180G / Y215S genes constructed according to the method of Example 1; Genetically engineered bacteria were cultured overnight at 37°C in LB medium containing 100 μg / l ampicillin, and then cultured in LB fermentation liquid medium containing 100 μg / l ampicillin at 37°C until OD 600 = 0.6, lower the temperature to 20°C for culture, add the inducer IPTG with a final concentration of 0.1 mM to induce culture, and centrifuge at 72 hours to obtain the supernatant enzyme solution, which is the crude enzyme solution.
Embodiment 3
[0035]Purification of embodiment 3 keratinase mutants and specific enzyme activity and thermostability enzymatic property determination
[0036] (1) The recombinant Escherichia coli containing the plasmid of the mutant gene was induced and cultured at 20° C. for 3 days to obtain a crude enzyme solution.
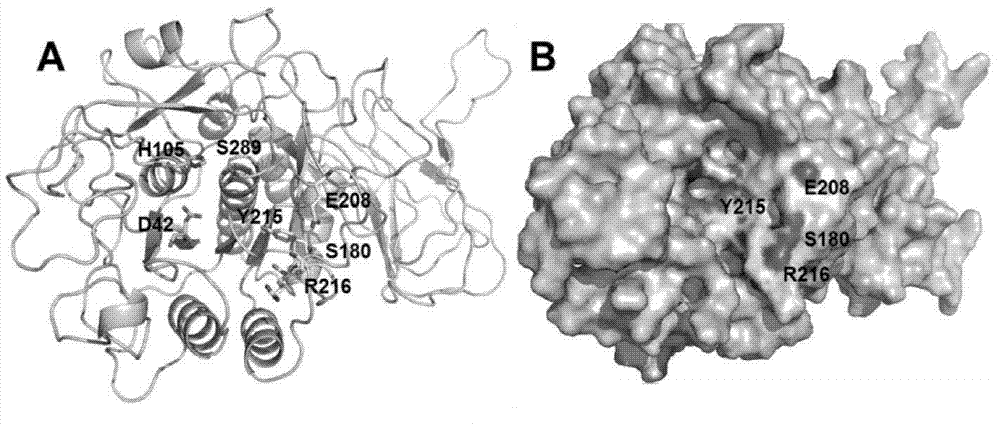
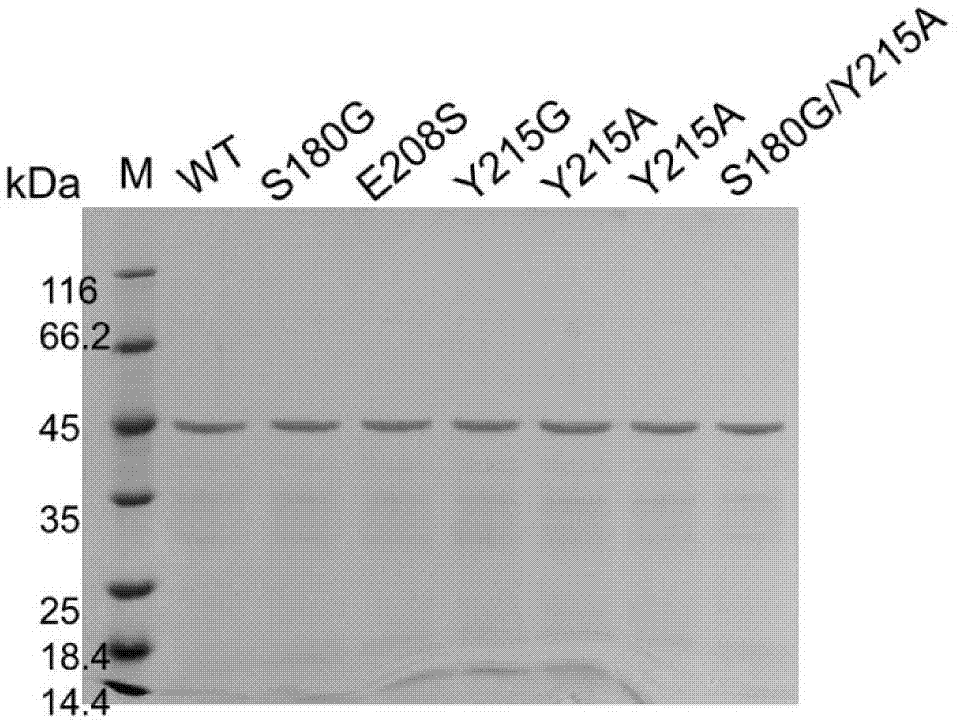
[0037] (2) Purify keratinase KerSMD with a purity of more than 90% and various mutants thereof from the crude enzyme solution by using an AKTA protein purifier (GE Company of the United States) and a nickel column of HisTrap FF crude 1 ml. SDS-PAGE of keratinase see image 3 , the molecular weight of each protein is close to 46kDa.
[0038] (3) The purified mutant keratinase was added to 50 mM Gly-NaOH buffer (pH 9.0), incubated at 60° C. for different times, and the enzyme activity was determined. Taking the initial unheated keratinase as 100% residual enzyme activity, the percentage value of the enzymatic activity measured later compared to the initial enzymatic activity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com