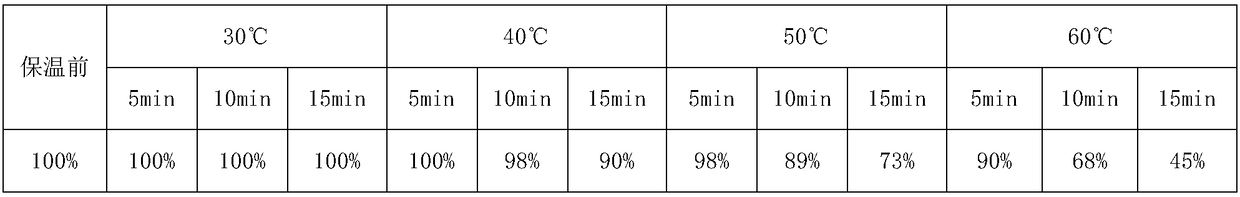
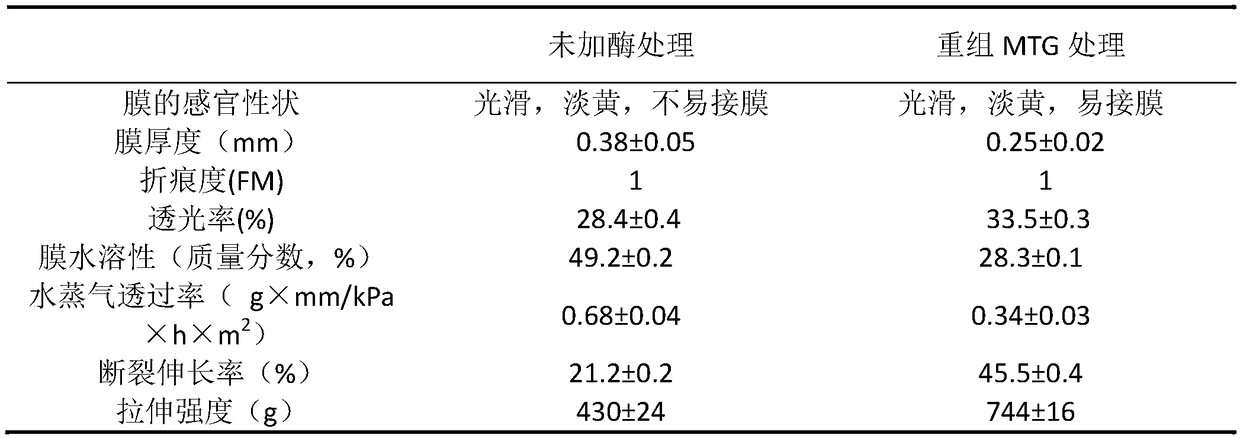
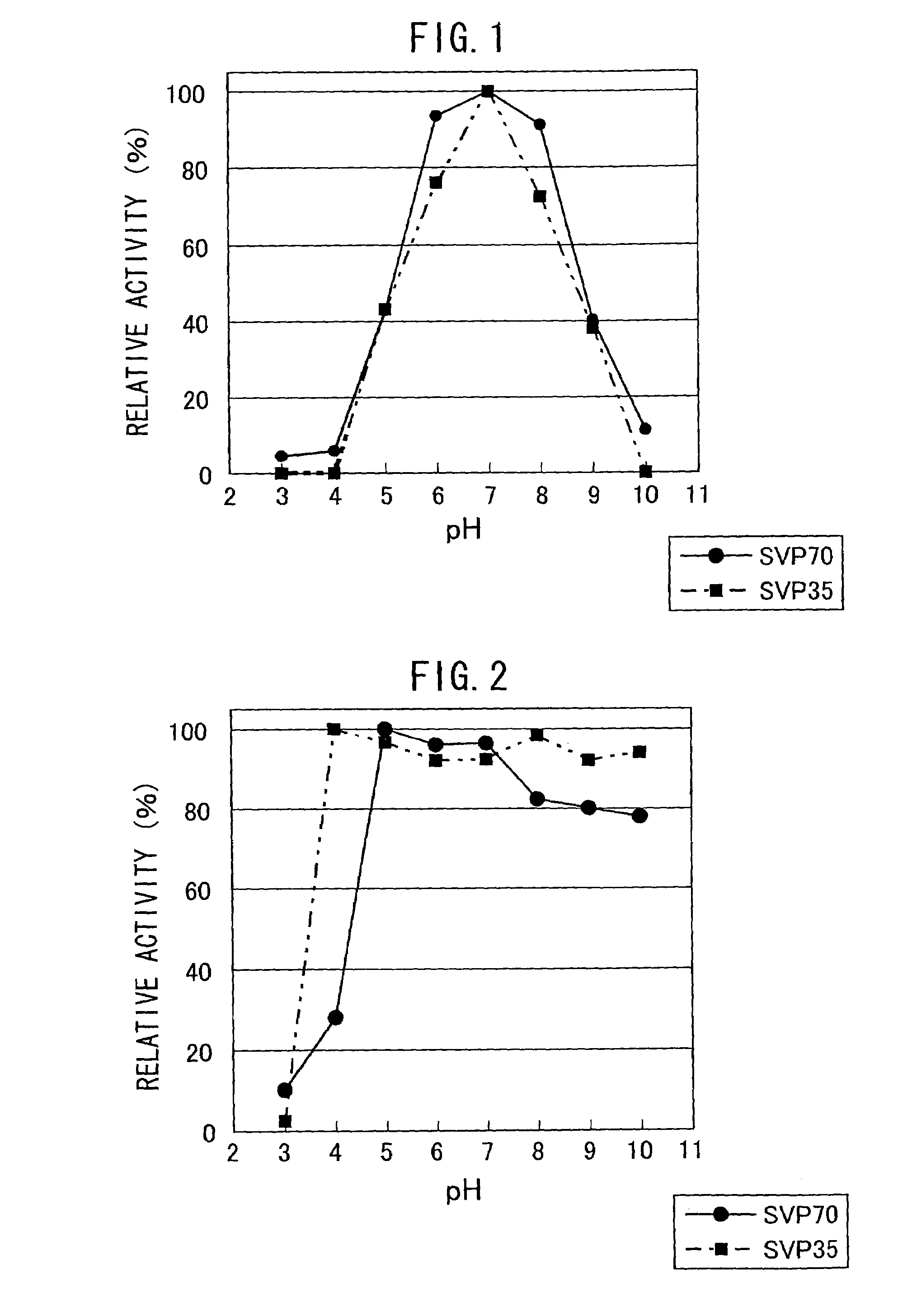
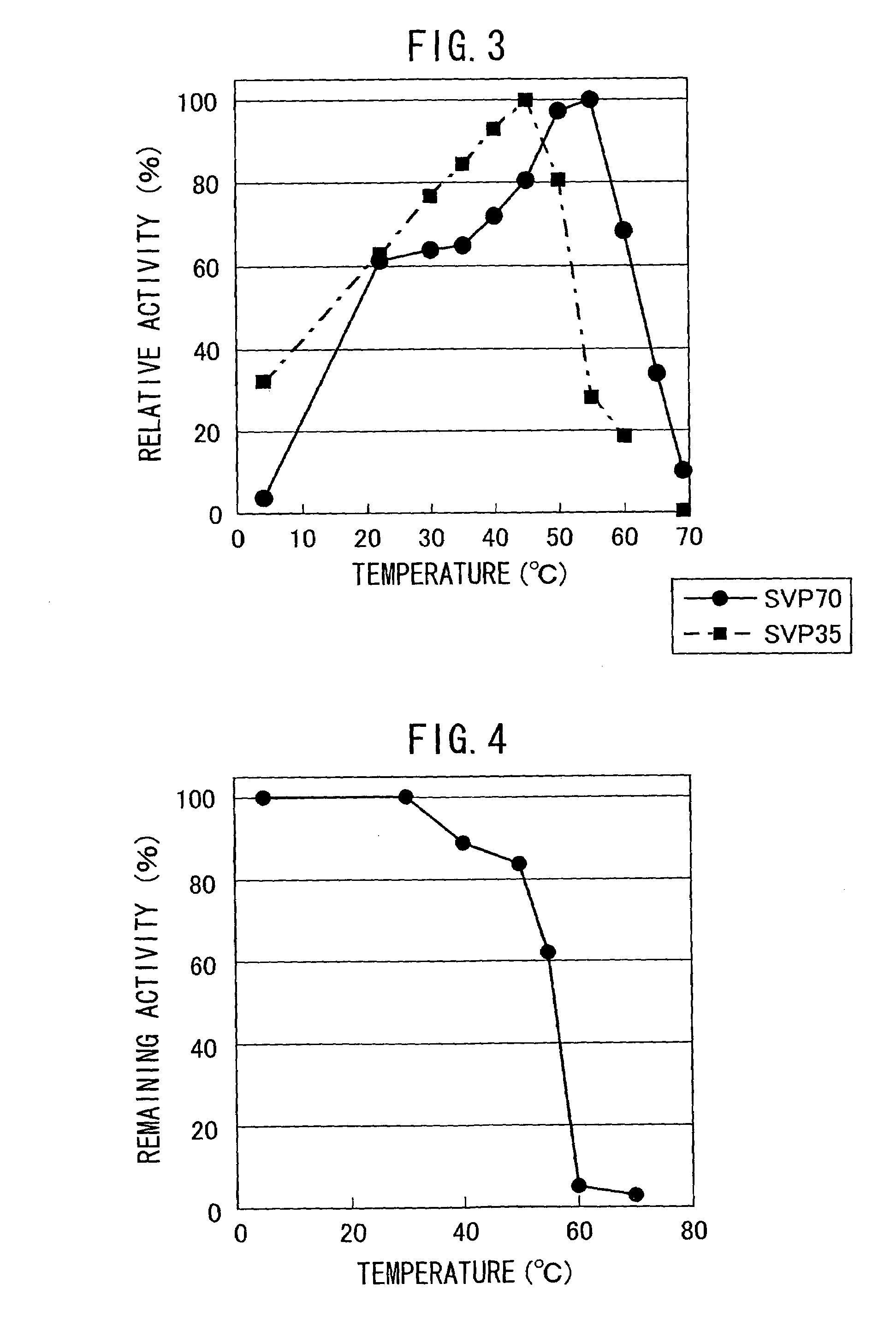
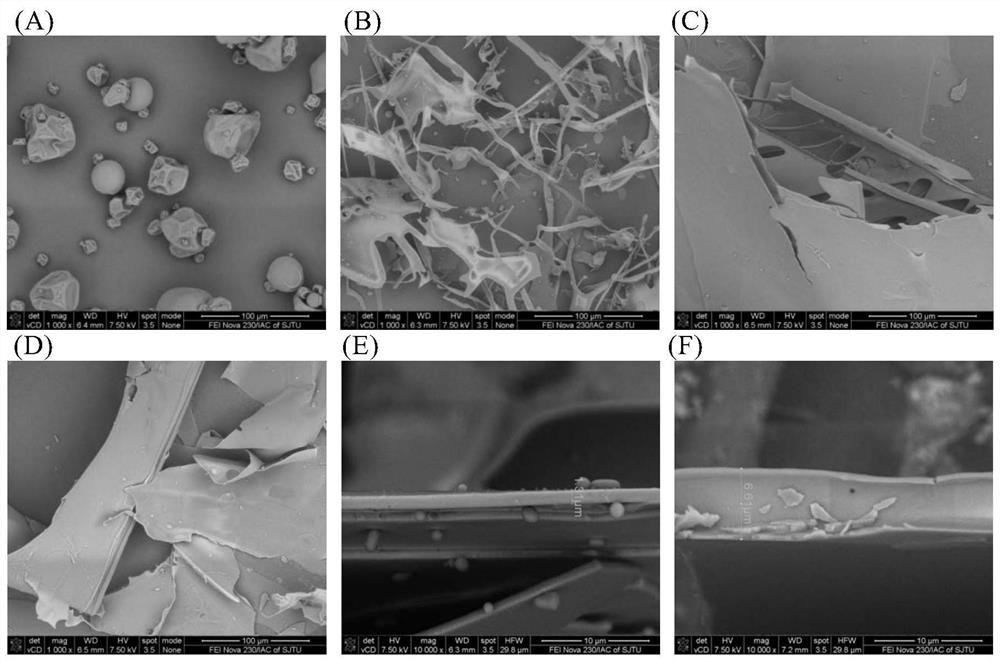
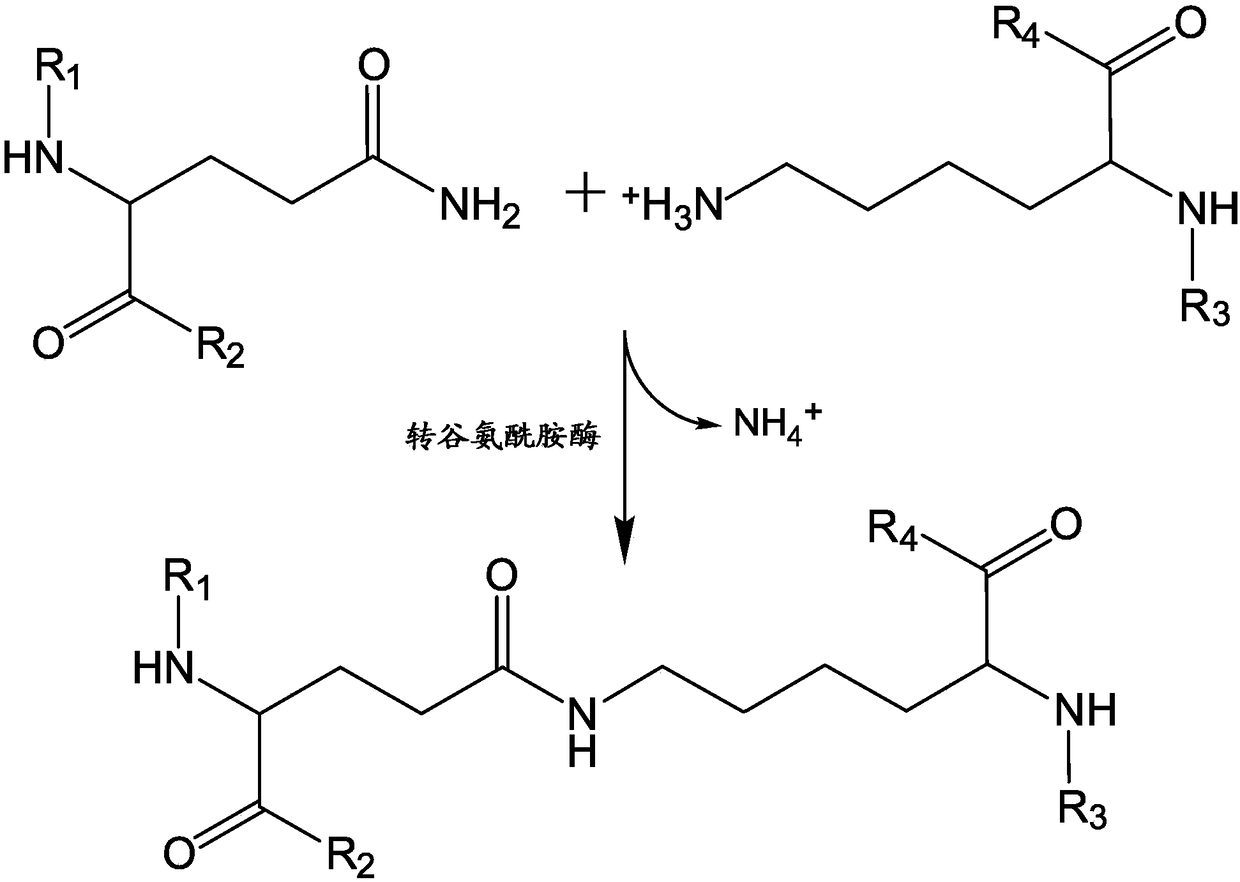
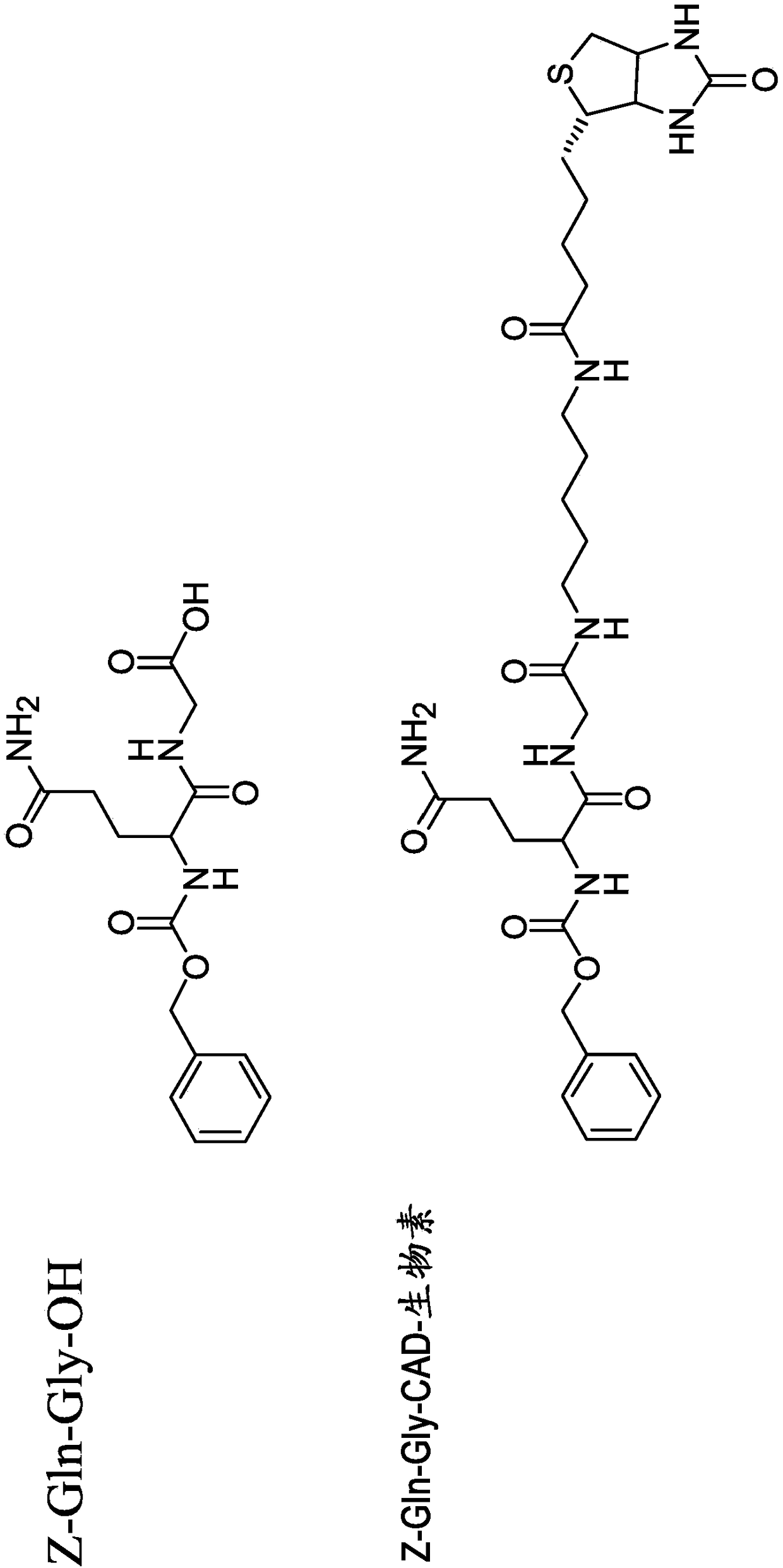
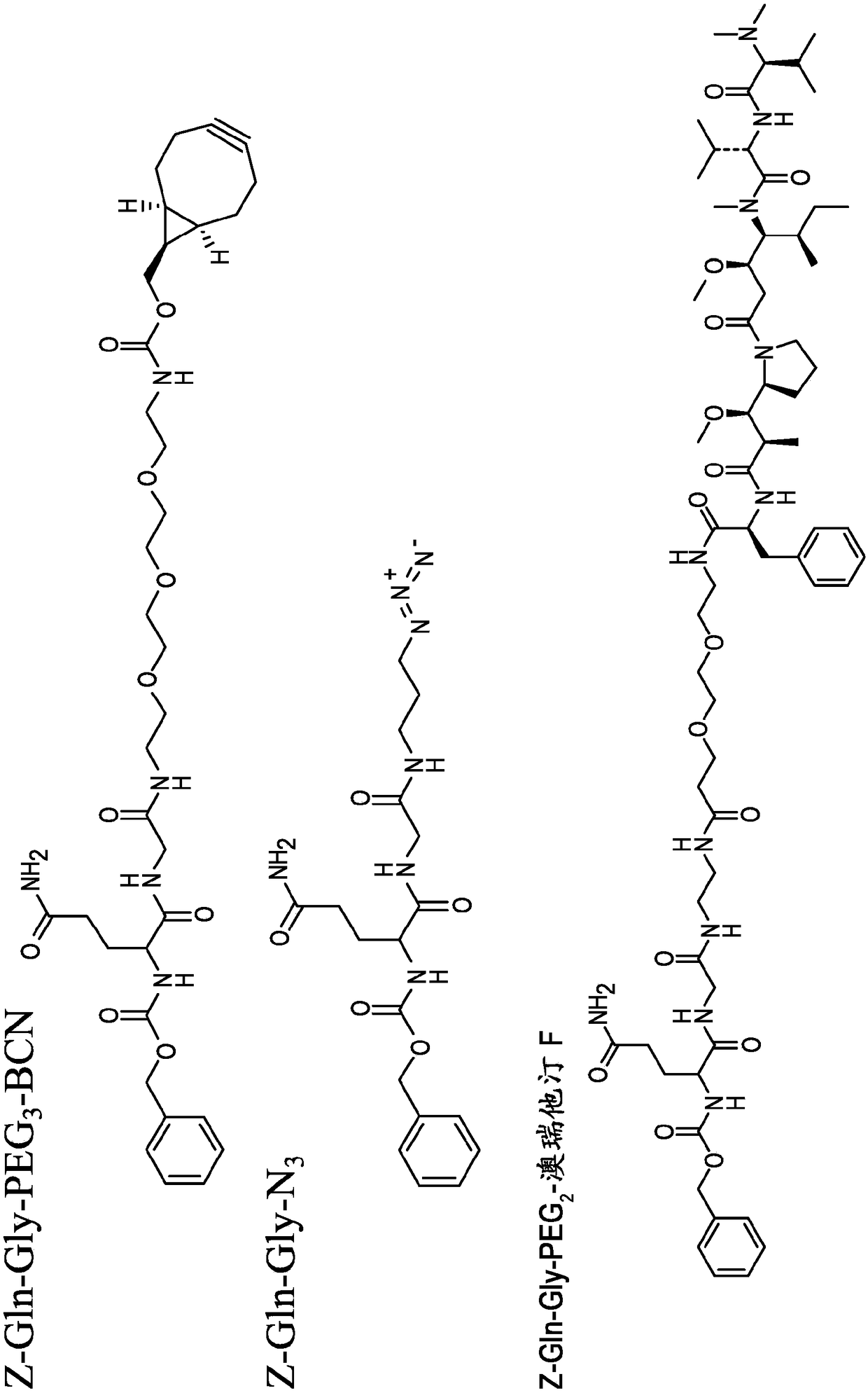
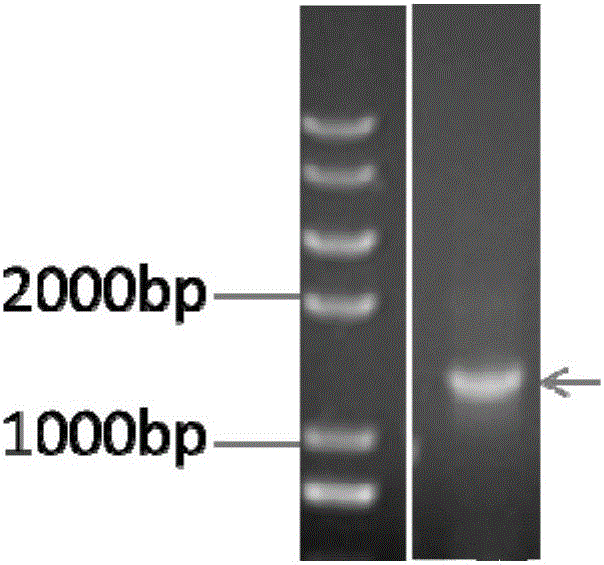
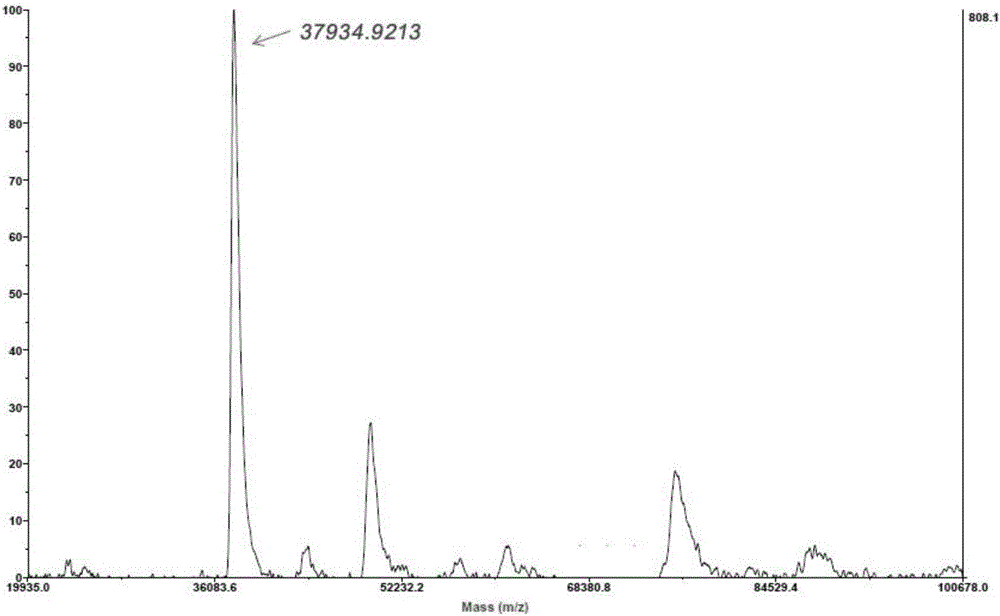
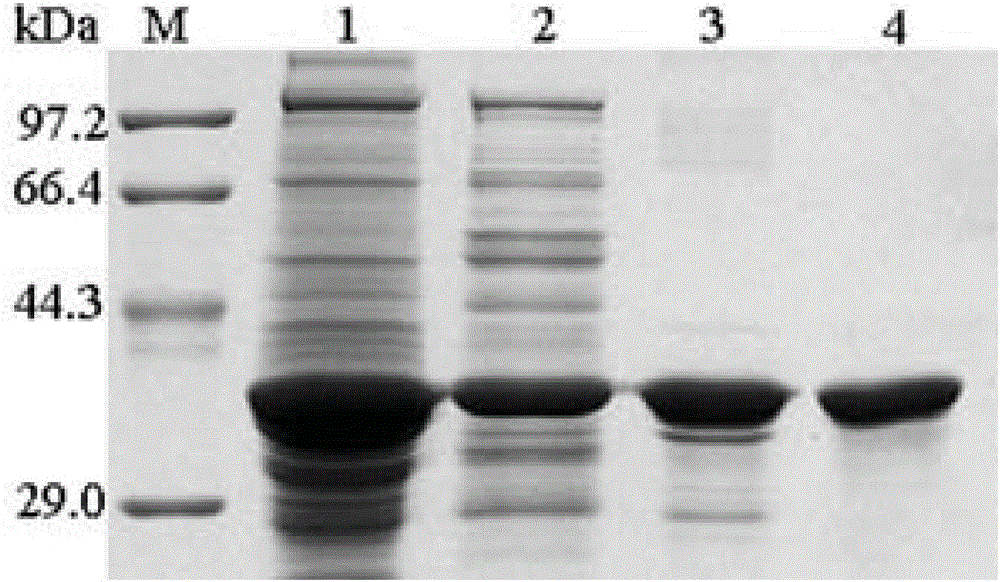
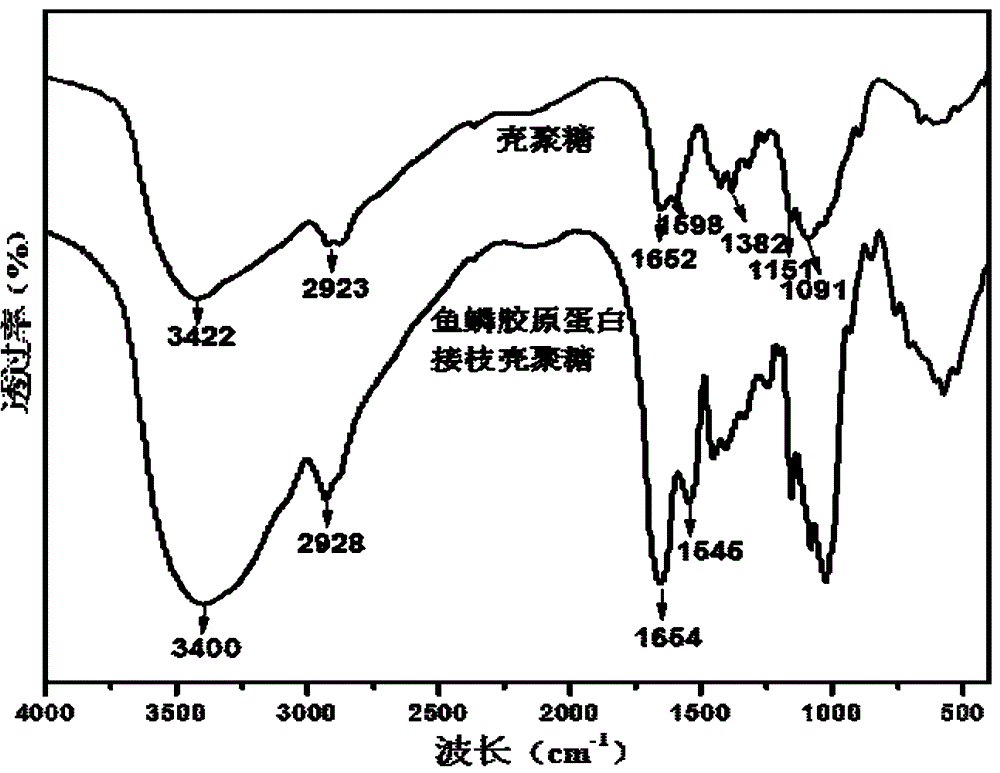
Patents
Literature
62 results about "Microbial transglutaminase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of using no-rinsing minced fillet of transglutaminase
InactiveCN101664208ANo pollution in the processOmit the "rinse" stepFood preparationSucroseMicrobial transglutaminase
A preparation method of using no-rinsing minced fillet of transglutaminase comprises the following steps: removing scale, skin and internal organs of material fish, simply washing, obtaining the minced fillet after separation by a flesh separator, adding sucrose, sorbitol, sodium tripolyph-osphate and focal sodium as antifreezers, adding microbial transglutaminase, stirring at low temperature, packing for shaping, after freezing at temperature of minus 35 DEG C, refrigerating in a cold store with temperature minus 18 DEG C, and the frozen minced fillet without rinsing is the same as the rinsedminced fillet by common method in quality.
Owner:EAST CHINA NORMAL UNIV +1
Transglutaminase Crosslinked Collagen Biomaterial for Medical Implant Materials
InactiveUS20080305517A1Enhanced ability to support cell attachment cellEnhanced ability to support attachmentBone implantTissue cultureCross-linkMicroorganism
The present invention provides a method for producing an improved biomaterial comprising treating a collagen biomaterial with a transglutaminase under conditions which permit the formation of cross-links within the collagen. Preferably, the transglutaminase is a tissue transglutaminase, a plasma transglutaminase or a microbial transglutaminase. In a preferred embodiment, the collagen biomaterial further comprises a cell adhesion factor, such as fibronectin. The invention further provides biomaterials obtainable by the methods of the invention, and medical implants and wound dressings comprising the same.
Owner:ASTON UNIV
Protein-grafted natural polysaccharide, preparation method and application thereof
The invention discloses protein-grafted natural polysaccharide, a preparation method and an application thereof, and concretely relates to bio-protein-grafted chitosan oligosaccharide, a preparation method and an application, which belongs to the high-molecular modification field. The bio-protein-grafted chitosan oligosaccharide is obtained by dissolved chitosan oligosaccharide and a bio-protein solution under effect of a catalyst microbial transglutaminase. According to the invention, reaction condition is mild, technology is simple, and the reaction substances are natural macromolecule with characteristics of environmental protection. The prepared water-soluble chitosan oligosaccharide bio-protein copolymer has good film forming ability, moisture-absorption effect, moisture-retention effect and antioxidation effect.
Owner:湖南尔康明胶有限公司
Glutamine transaminase with improved heat stability and application thereof
ActiveCN102994469AGood enzymatic propertiesImprove thermal stabilityBacteriaTransferasesEscherichia coliMicrobial transglutaminase
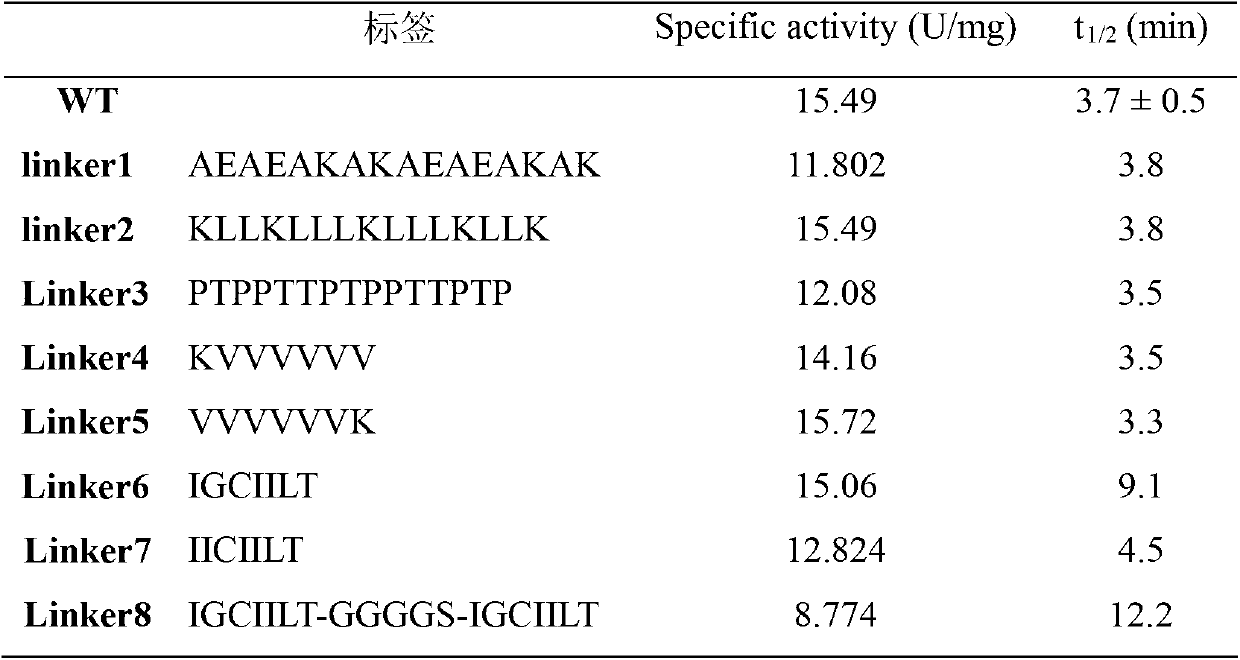
The invention discloses a glutamine transaminase with improved heat stability and application thereof. High-efficiency expression of microbial transglutaminase (MTG) in escherichia coli serves as a modification platform, a label facilitating improvement of heat stability is added at the C end of MTG maturase, preference is given to IGCIILT to obtain a mutant strain with good enzymatic property, and the heat stability is improved by 2.5 times and 3 times. Modified enzyme is suitable for industrial application, the production cost can be reduced, and the production efficiency can be improved.
Owner:TAIXING YIMING BIOLOGICAL PRODS
Rapid hemostatic product for war wounds and preparation method thereof
ActiveCN103816562AImprove recovery effectImprove heat resistancePeptide/protein ingredientsCatheterBiotechnologySide effect
The invention discloses a rapid hemostatic product for war wounds, as well as a preparation method and application thereof. The rapid hemostatic product comprises a substrate with added microbial transglutaminase (mTG), wherein the amino acid sequence of the transglutaminase is the protein sequence coded by the gene shown in SEQ ID NO.: 3, or a protein sequence shown in SEQ ID NO.: 4. More specifically, the rapid hemostatic product is selected from a hemostatic agent, a hemostatic device, a hemostatic first-aid kit or a common hemostatic product, the substrate is in a fluid or semi-solid form, which can be absorbed by a human body, or in a malleable gel or colloid form, and the rapid hemostatic product contains the microbial transglutaminase and gelatin. The rapid hemostatic product has the advantages of rapid hemostasis, convenience for use, organ adhesion prevention, wound healing promotion, low toxic and side effects, complete absorption and the like; except for hemostasis in a war, the rapid hemostatic product can also be widely applied to rapid hemostasis for wounds in fire control, accidents and other wild environments.
Owner:EAST CHINA NORMAL UNIVERSITY
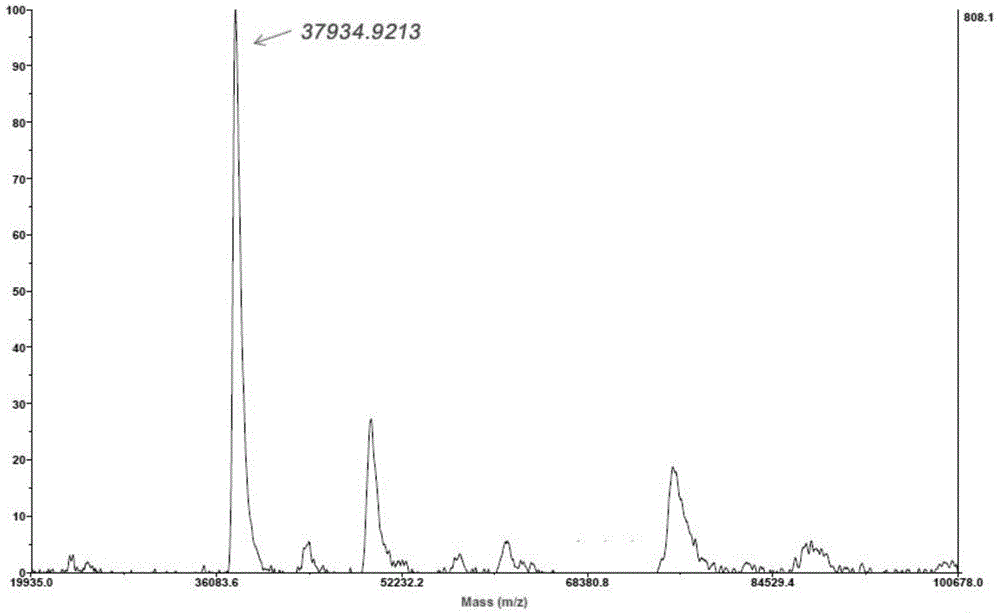
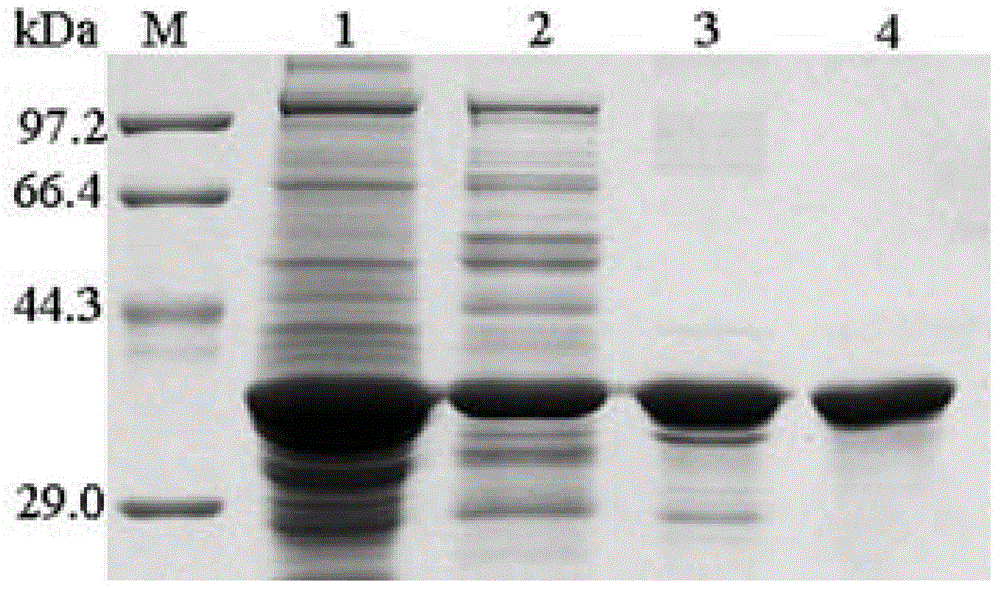
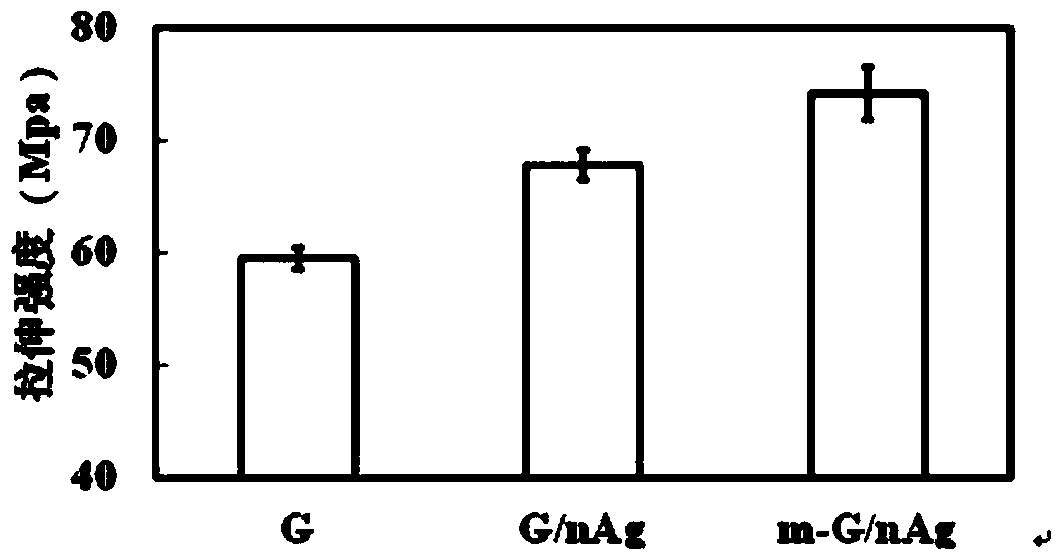
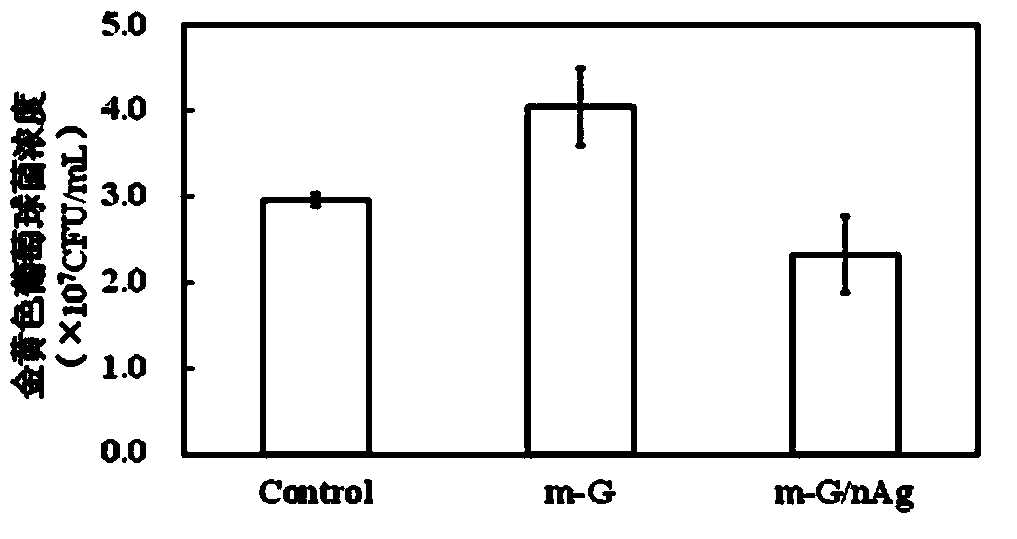
Gelatin/nano silver composite material decorated by microbial transglutaminase as well as preparation method and application thereof
InactiveCN103360613AImprove mechanical propertiesImprove stabilityAbsorbent padsProsthesisNon toxicityBiocompatibility Testing
The invention belongs to the field of biological materials and interdiscipline of polymer chemistry and biochemistry and discloses a gelatin / nano silver composite material decorated by microbial transglutaminase and a preparation method and an application thereof. The gelatin / nano silver composite material decorated by microbial transglutaminase is prepared by utilizing a gelatin / nano silver composite material as main material and utilizing the microbial transglutaminase as a modifier, the material has good biocompatibility, non-toxicity and strong antibacterial activity, the mechanical properties and water stability of the material are good, and meanwhile, the material is simple in preparation process, mild in reaction conditions, easy to control and low in production cost, thereby having good application prospect and very extensive usage in biomedical materials and biomedicine field, such as antibacterial and biodegradable skin dressing capable of reducing infection and inflammation and protecting wound, wound healing agent and artificial skin, and the like.
Owner:WUHAN UNIV OF TECH
Reinforcing liquid and reinforcing method for protecting ancient silk fabric cultural relics
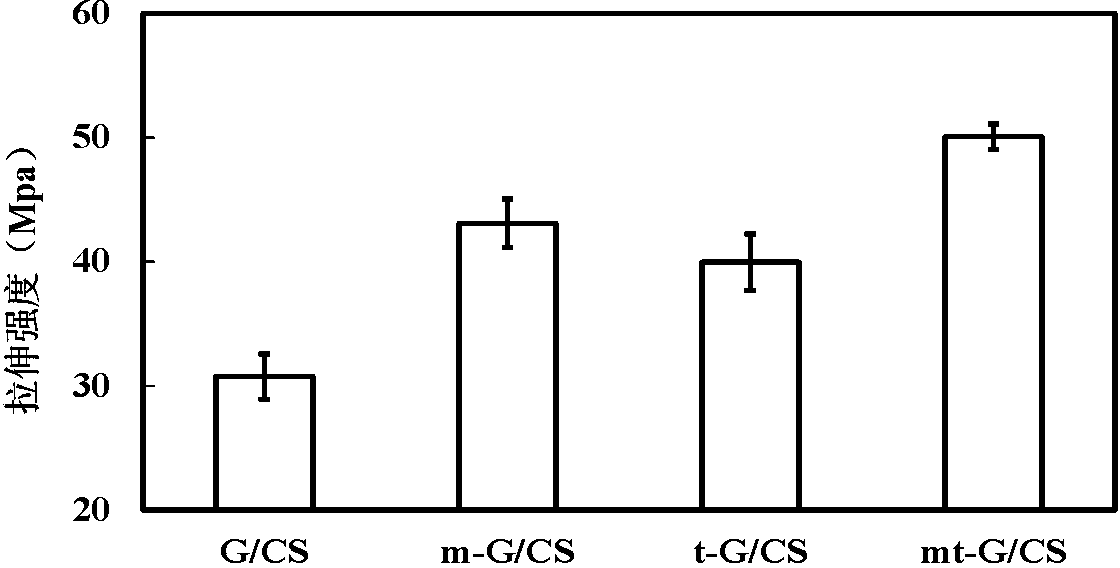
The invention discloses a reinforcing liquid for protecting ancient silk fabric cultural relics. The reinforcing liquid is a solution, which is formed by dissolving sodium caseinate and glutamine transaminase in a Tris-HCl buffering liquid, wherein the mass concentration of the sodium caseinate is 0.5-3.0%, the ratio between enzyme activity of the glutamine transaminase and mass of the sodium caseinate is 5-50 U / g; the pH value of the Tris-HCl buffering liquid is 6.0-8.0, and the concentration of the Tris-HCl buffering liquid is 0.05-0.5 M. According to the invention, as the sodium caseinate is catalyzed through the adoption of an MTG (Microbial Transglutaminase) enzyme to be polymerized on a silk fabric cultural relic main body so as to generate proteins with large molecular weights and a net-shaped structure is formed in a silk fiber, the mechanical strength is enhanced; and it is proved by experiments that a good effect of reinforcing silk fabric materials is achieved.
Owner:UNIV OF SCI & TECH OF CHINA
Method for separating and purifying microbial transglutaminase
InactiveCN102776160AHigh puritySatisfied with medical hemostatic materialsTransferasesMicroorganismMicrobial transglutaminase
The invention discloses a method for separating and purifying microbial transglutaminase. The method comprisies the following steps: adding 50-200mg / L dispase in streptomycete fermented liquid, subjecting to ethanol depositing, anion exchange chromatography and cation exchange chromatography, de-salting, concentrating, filtering, and freezing to be dry, so as to obtain the microbial transglutaminase, wherein the purity of the microbial transglutaminase is higher than 95% and the specific activity is 20000-50000U / g. The method disclosed by the invention is simple and is capable of obtaining high-purity transglutaminase from microbial fermentation product with the yield of higher than 70%.
Owner:EAST CHINA NORMAL UNIV
Injectable porous hydrogels
PendingUS20200254142A1Effective timePharmaceutical delivery mechanismProsthesisMicrobial transglutaminaseGlutaminase
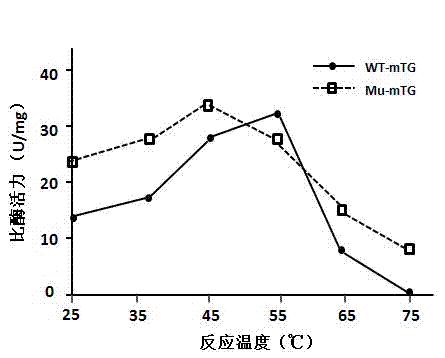
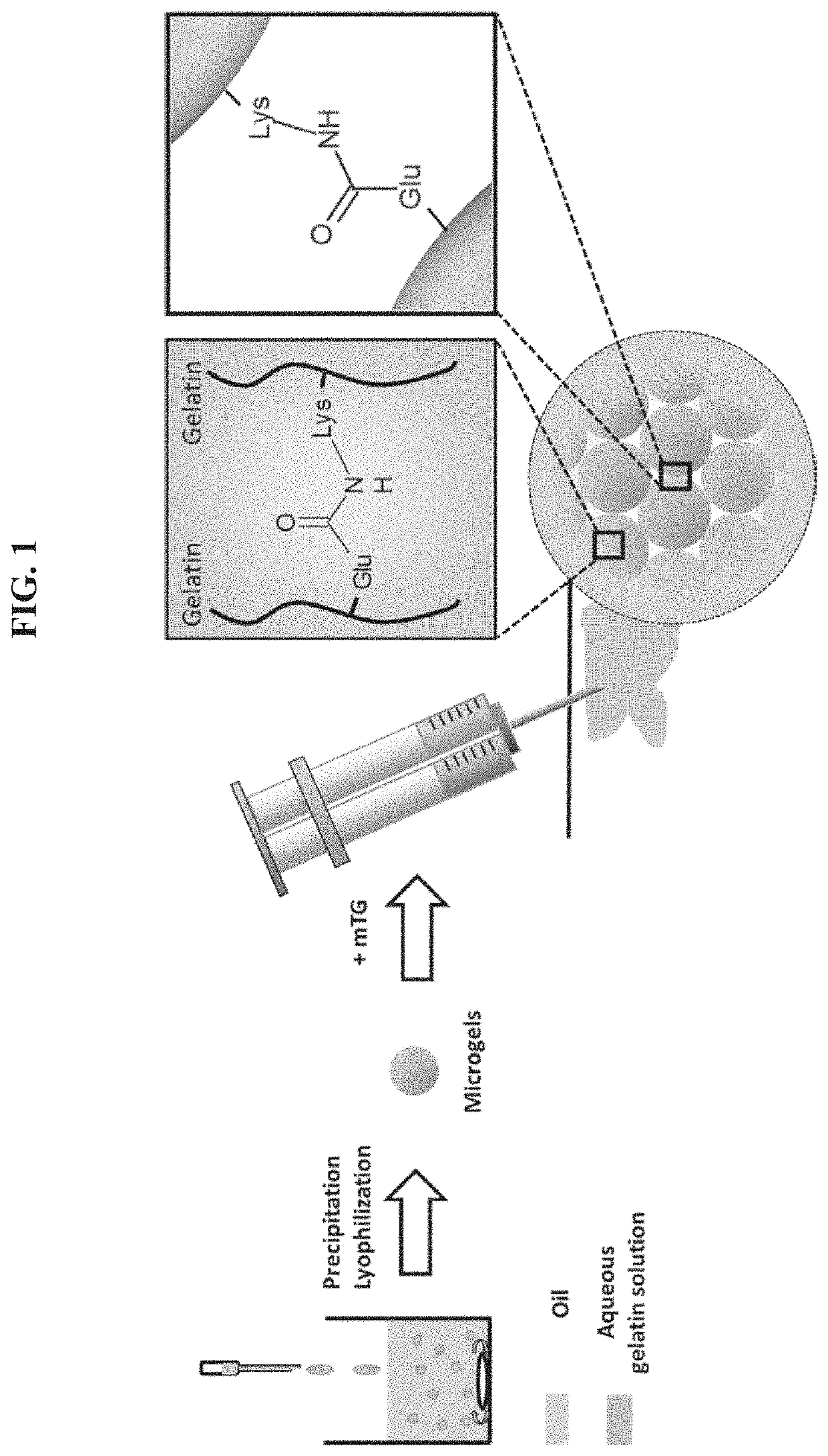
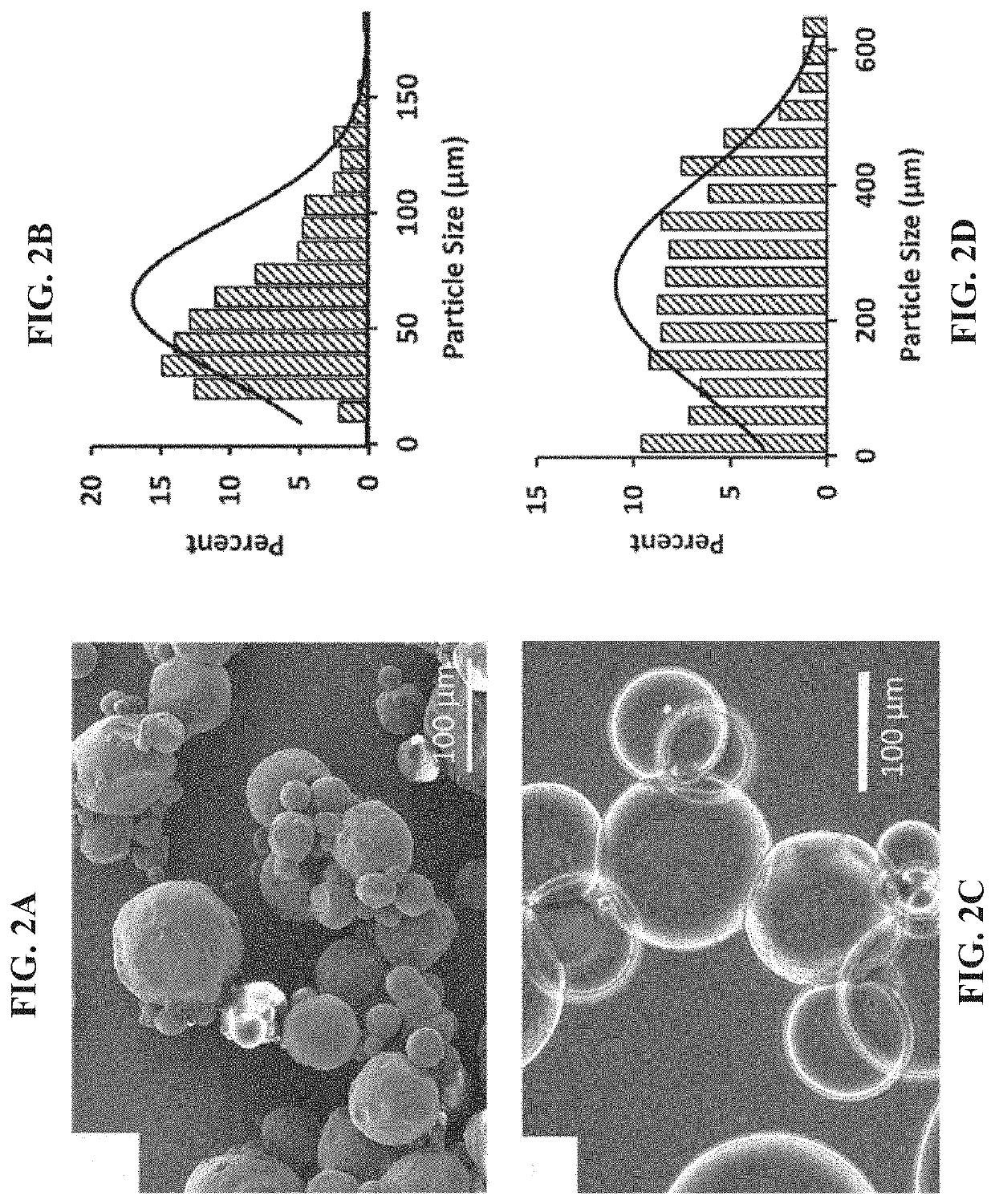
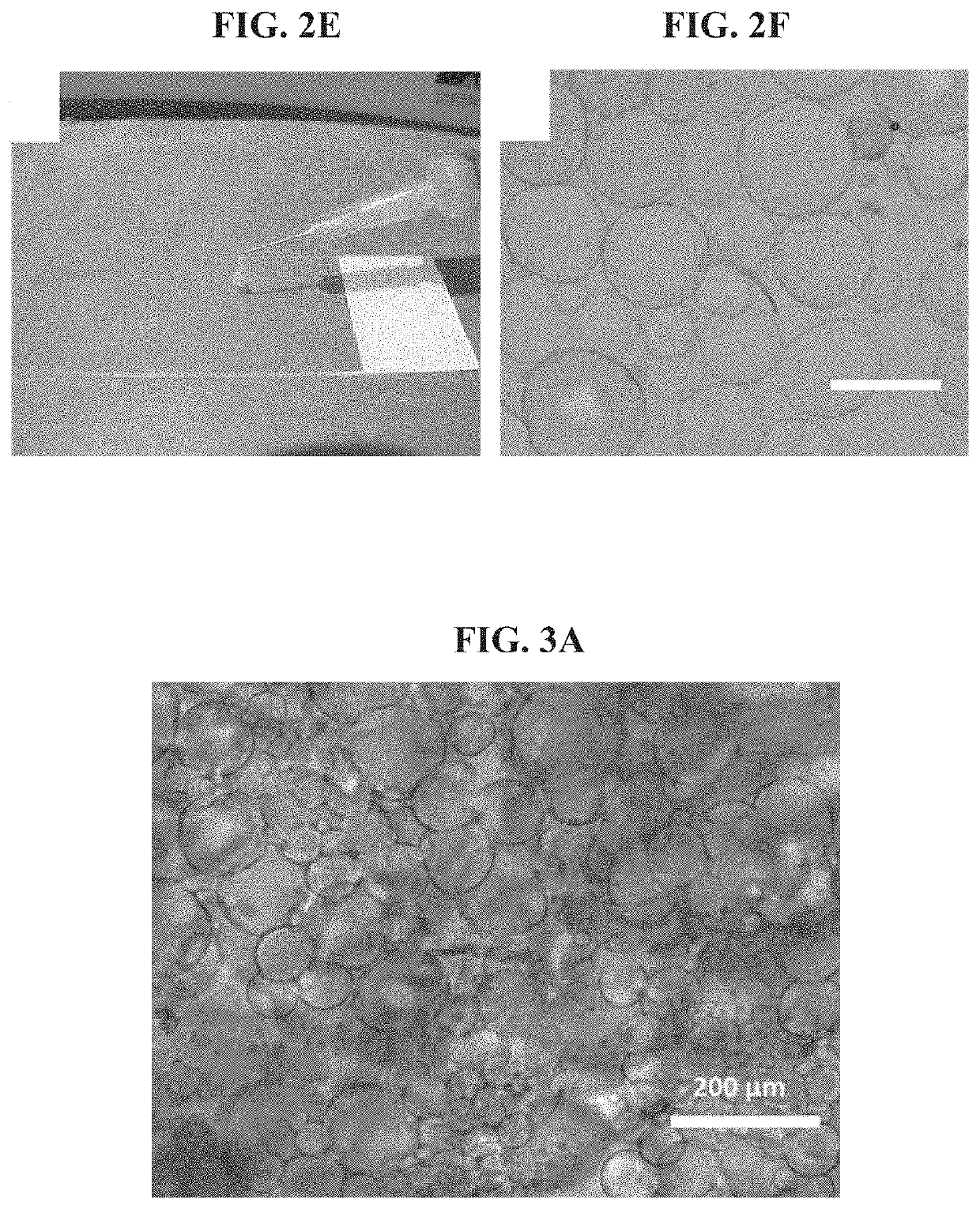
Injectable macroporous hydrogels, and methods of producing same, are described. The injectable macroporous hydrogels may be formed by mixing gelatin microgels with an enzyme. In at least some embodiments, the enzyme may be transglutaminase, and more specifically microbial transglutaminase (mTG).
Owner:UNIVERSITY OF NEW HAMPSHIRE
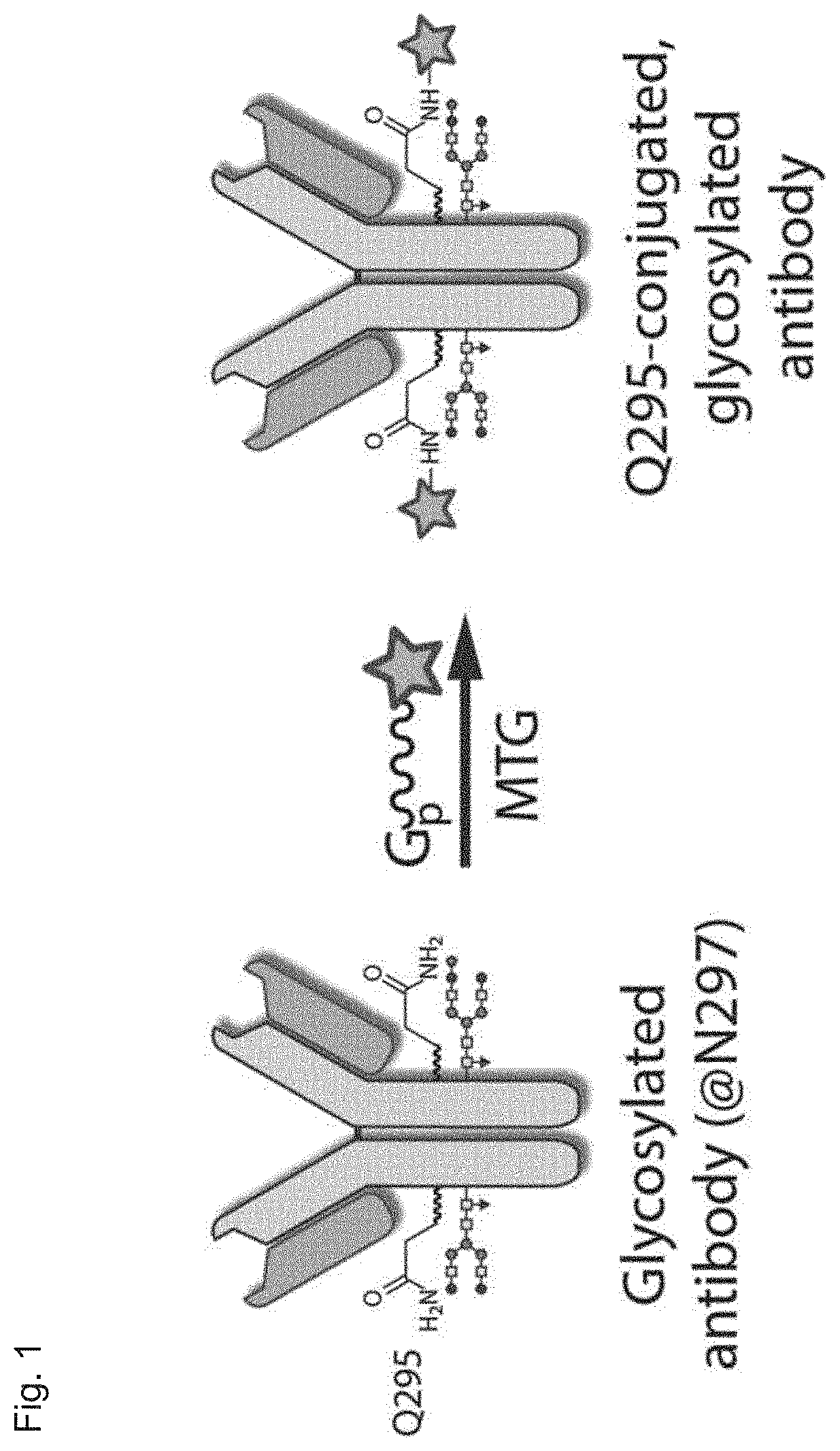
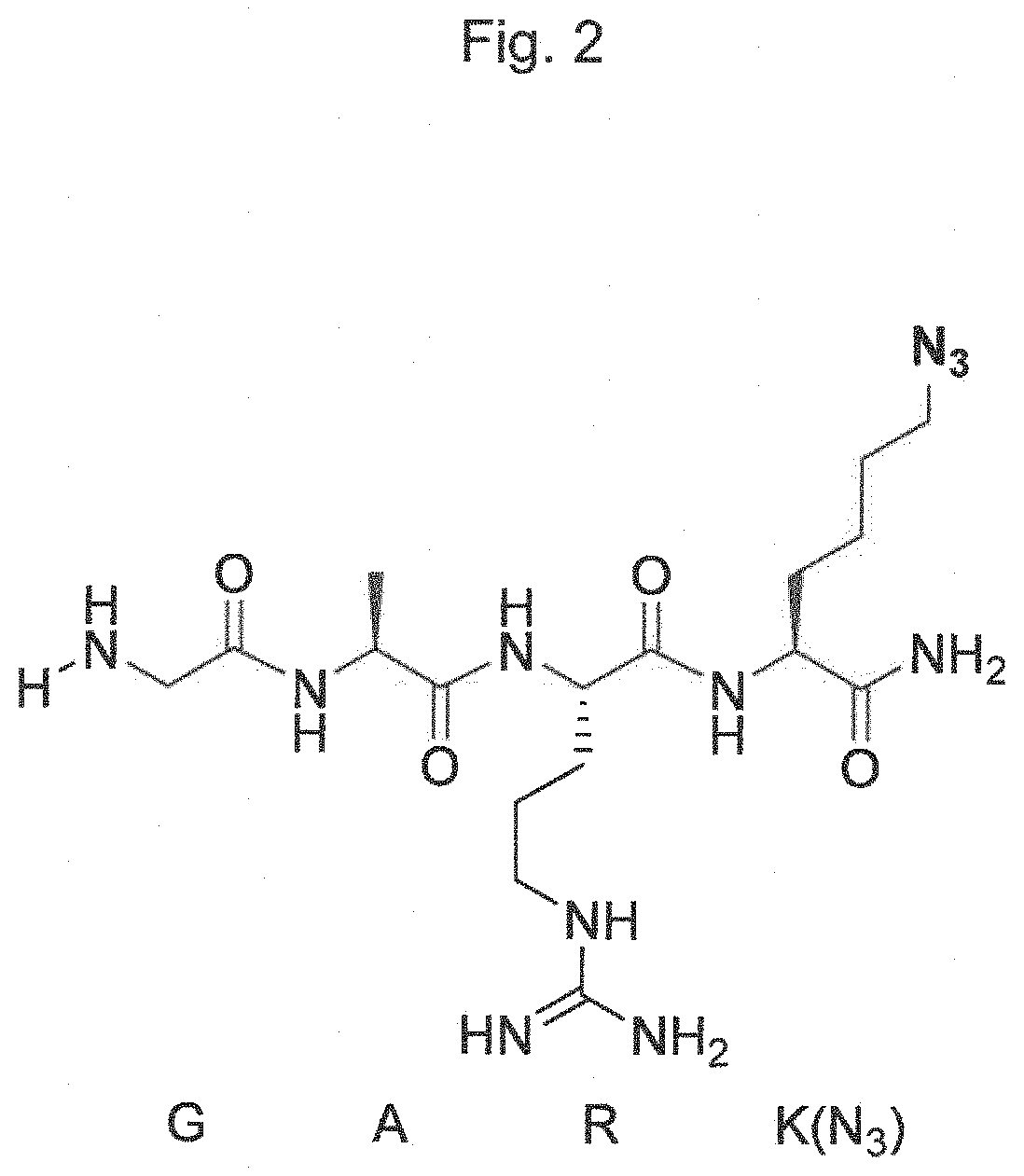
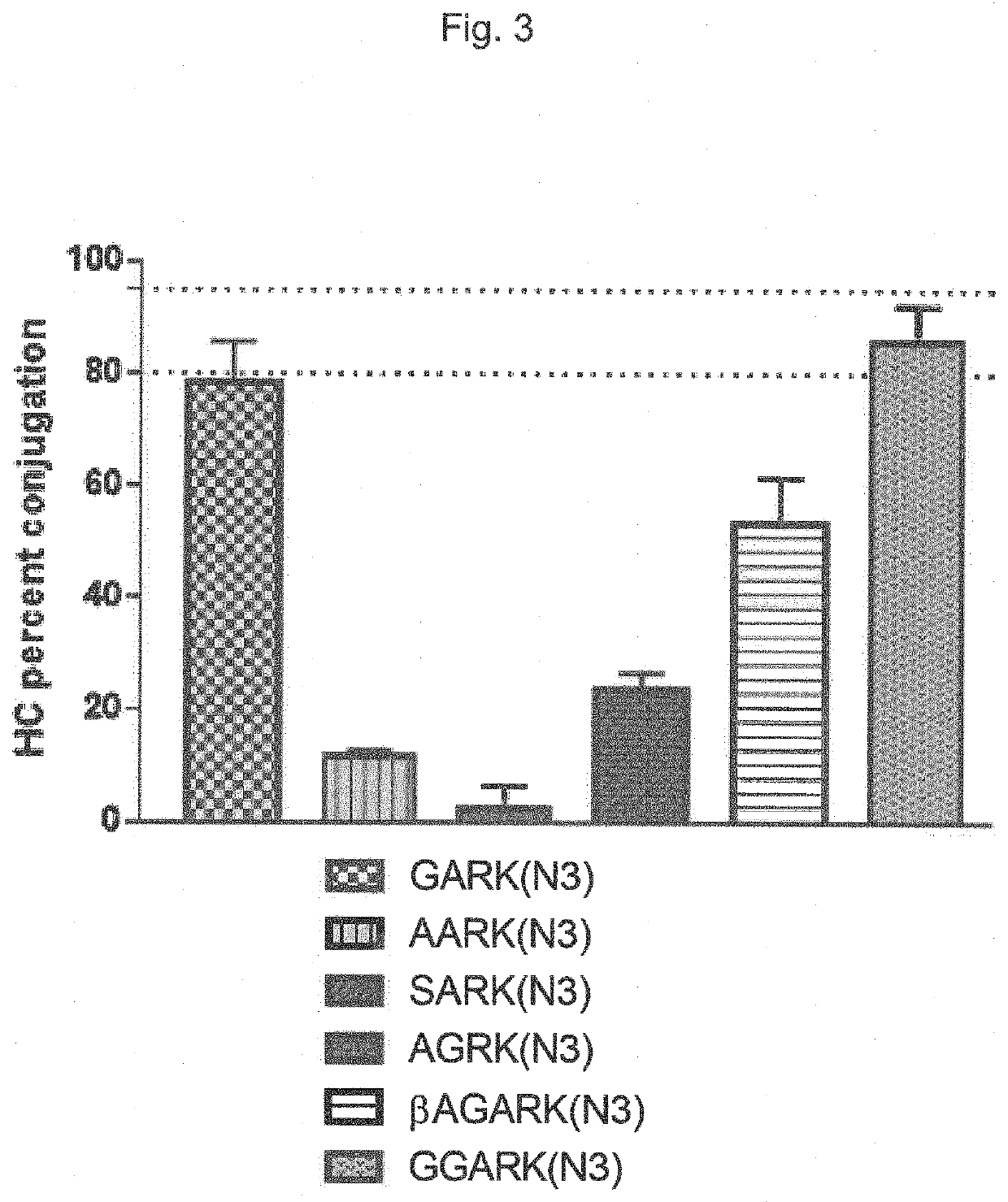
Transglutaminase conjugation method with a glycine based linker
The present invention relates to a method for generating an antibody-payload conjugate by means of a microbial transglutaminase (MTG). The method comprises a step of conjugating a linker comprising or having the peptide structure (shown in N->C direction) Gly-(Aax)m-B-(Aax)n via the N-terminal primary amine of the N-terminal glycine (Gly) residue to a glutamine (Gln) residue comprised in the heavy or light chain of an antibody.
Owner:PAUL SCHERRER INSTITUT
Protein-transglutaminase (TG) reinforcement method of fragile wool fabrics
ActiveCN103572600ADoes not affect appearanceObvious superiorityBiochemical fibre treatmentAnimal fibresMicrobial transglutaminaseAging resistance
The invention relates to a protein-transglutaminase (TG) reinforcement method of fragile wool fabrics. The method comprises the steps of firstly, dipping the fragile wool fabrics into a keratin solution; dipping the fragile wool fabrics into a TG solution; spreading out the dipped fragile wool fabrics, and carrying out reaction on the fragile wool fabrics for 8-4h at the temperature of 50-60 DEG C; finally, washing the fragile wool fabrics for three times by water, spreading out the washed fragile wool fabrics, and drying the wool fabrics at a shade place. The protein-TG reinforcement method has the beneficial effects that under the help of microbial transglutaminase (MTG), keratin is used for reinforcing the fractured polypeptide chains, so that the appearances of wool fabric cultural relics are not influenced, and the method has prominent superiority in the aspect of high temperature aging resistance.
Owner:ZHEJIANG SCI-TECH UNIV
Method for improving gel coagulation property of milk protein concentrates and application of method
InactiveCN106720302AHigh gel strengthImprove water holding capacityMilk preparationMicroorganismMicrobial transglutaminase
The invention relates to a method for improving the gel coagulation property of milk protein concentrates and an application of the method. According to the method disclosed by the invention, the gel coagulation property of the milk protein concentrates is improved by treating the milk protein concentrates through a microorganism namely transglutaminase. By controlling the time of a crosslinking reaction to be 1-2h, controlling the reaction temperature to be 33-35 DEG C, controlling the pH to be 7.0-7.5 and controlling the addition quantity of enzymes in protein to be 2.0-2.5U / g, the modified milk protein concentrates with good gel coagulation intensity and good water-holding capacity are obtained. Compared with ordinary milk protein concentrates, the milk protein concentrates under the condition have the advantages that the gel coagulation intensity is improved by 150-206%, and the water-holding capacity is improved by 50-90%. The modified milk protein concentrates are applied to the preparation of stirring type yoghourt, and under the premise that the fermentation time of the yoghourt is not obviously changed, the gel coagulation intensity and the water-holding capacity of the stirring type yoghourt can be notably improved.
Owner:CHINA AGRI UNIV
Method of producing microbial transglutaminase
ActiveUS20100159560A1Little degradationHydrolasesTransferasesMicroorganismMicrobial transglutaminase
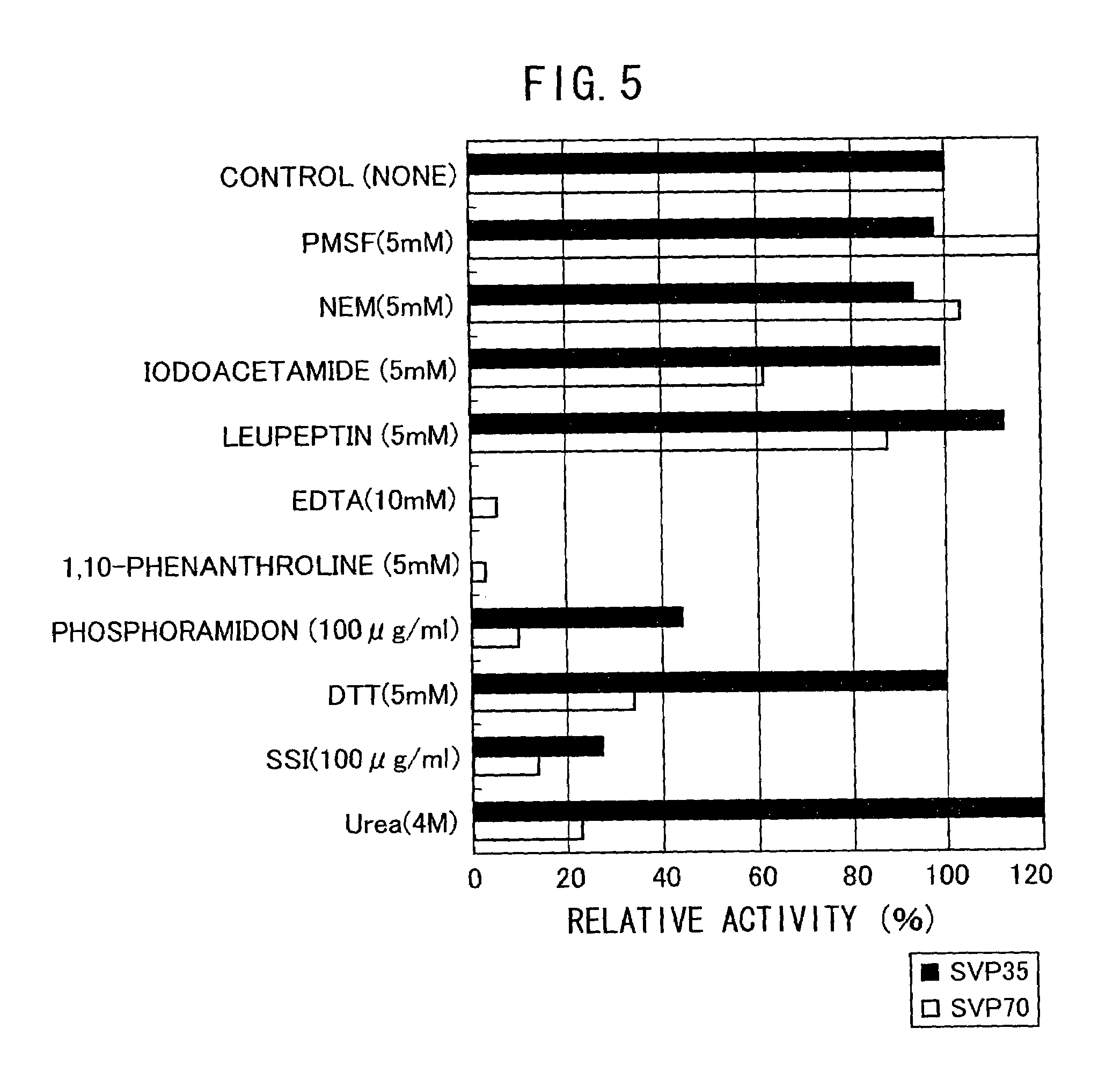
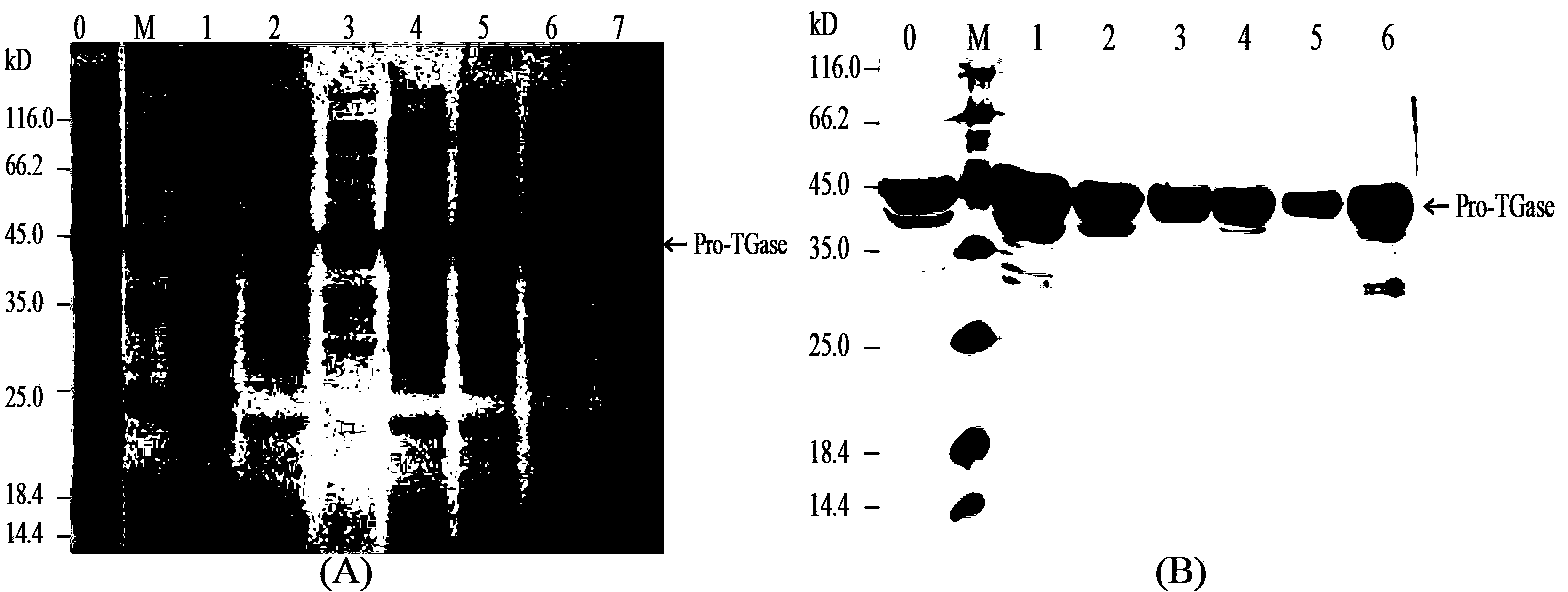
The present invention provides a neutral metalloprotease from actinomycetes which selectively cleaves a pro-structure part of a microbial protransglutaminase and a gene encoding said neutral metalloprotease. An active microbial transglutaminase having the pro-structure part cleaved can be obtained by culturing a microorganism into which a gene encoding the neutral metalloprotease from actinomycetes according to the present invention has been introduced, where by producing the neutral metalloprotease from actinomycetes, and reacting it on a microbial protransglutaminase.
Owner:AJINOMOTO CO INC
Microbe transglutaminase modified soya flour-based wood adhesive and preparation method thereof
InactiveCN101696347ANo pollutionIncrease incomeStarch adhesivesMacromolecular adhesive additivesAdhesiveGlutaminase
The invention discloses a microbe transglutaminase modified soya flour-based wood adhesive and a preparation method thereof, and relates to a wood adhesive and a preparation method thereof. The microbe transglutaminase modified soya flour-based wood adhesive solves the problems that the conventional formaldehyde-type adhesives constantly release formaldehyde, a mode of copolymerizing or blending a soy-based adhesive and synthetic resin improves the water resistance but causes existing volatile toxic substances, consumes a great deal of energy and pollutes environment, and isolated soy protein is adopted as a raw material to modify the soy-based adhesive to cause high cost. The adhesive of the invention is prepared from water, defatted soya flour and microbe transglutaminase. The preparation method comprises the following steps: 1, weighing raw materials; 2, after temperature rise of the water, adding the defatted soya flour, and stirring to prepare slurry; and 3, adjusting pH value of the slurry, adding the microbe transglutaminase, performing stirring reaction, adjusting pH value, stirring again, discharging, and cooling to room temperature. The adhesive has low formaldehyde release, low cost, low energy consumption and no environmental pollution in the using process.
Owner:NORTHEAST FORESTRY UNIVERSITY
Thermal-stable microbial transglutaminase, and coding gene thereof
InactiveCN108103041AExpand genetic resourcesImprove thermal stabilityTransferasesFermentationMicroorganismNucleotide
The invention discloses a thermal-stable microbial transglutaminase, and a coding gene thereof. The amino acid sequence of the thermal-stable microbial transglutaminase is represented by SEQ ID NO.1.The nucleotide sequence of the gene used for coding the thermal-stable microbial transglutaminase is represented by SEQ ID NO.2. The half life of the thermal-stable microbial transglutaminase at 60 DEG C is 10min or longer. The thermal-stable microbial transglutaminase is capable of enriching microbial transglutaminase gene resources, and providing foundation of enlargement of application range ofmicrobial transglutaminase in food processing.
Owner:TAIXING DONGSHENG FOOD TECH
Method of producing microbial transglutaminase
The present invention provides a neutral metalloprotease from actinomycetes which selectively cleaves a pro-structure part of a microbial protransglutaminase and a gene encoding said neutral metalloprotease. An active microbial transglutaminase having the pro-structure part cleaved can be obtained by culturing a microorganism into which a gene encoding the neutral metalloprotease from actinomycetes according to the present invention has been introduced, where by producing the neutral metalloprotease from actinomycetes, and reacting it on a microbial protransglutaminase.
Owner:AJINOMOTO CO INC
Glutamine transaminase with improved enzymatic activity and thermal stability
InactiveCN102660515BGood enzymatic propertiesImprove thermal stabilityTransferasesMicroorganism based processesEscherichia coliMicrobial transglutaminase
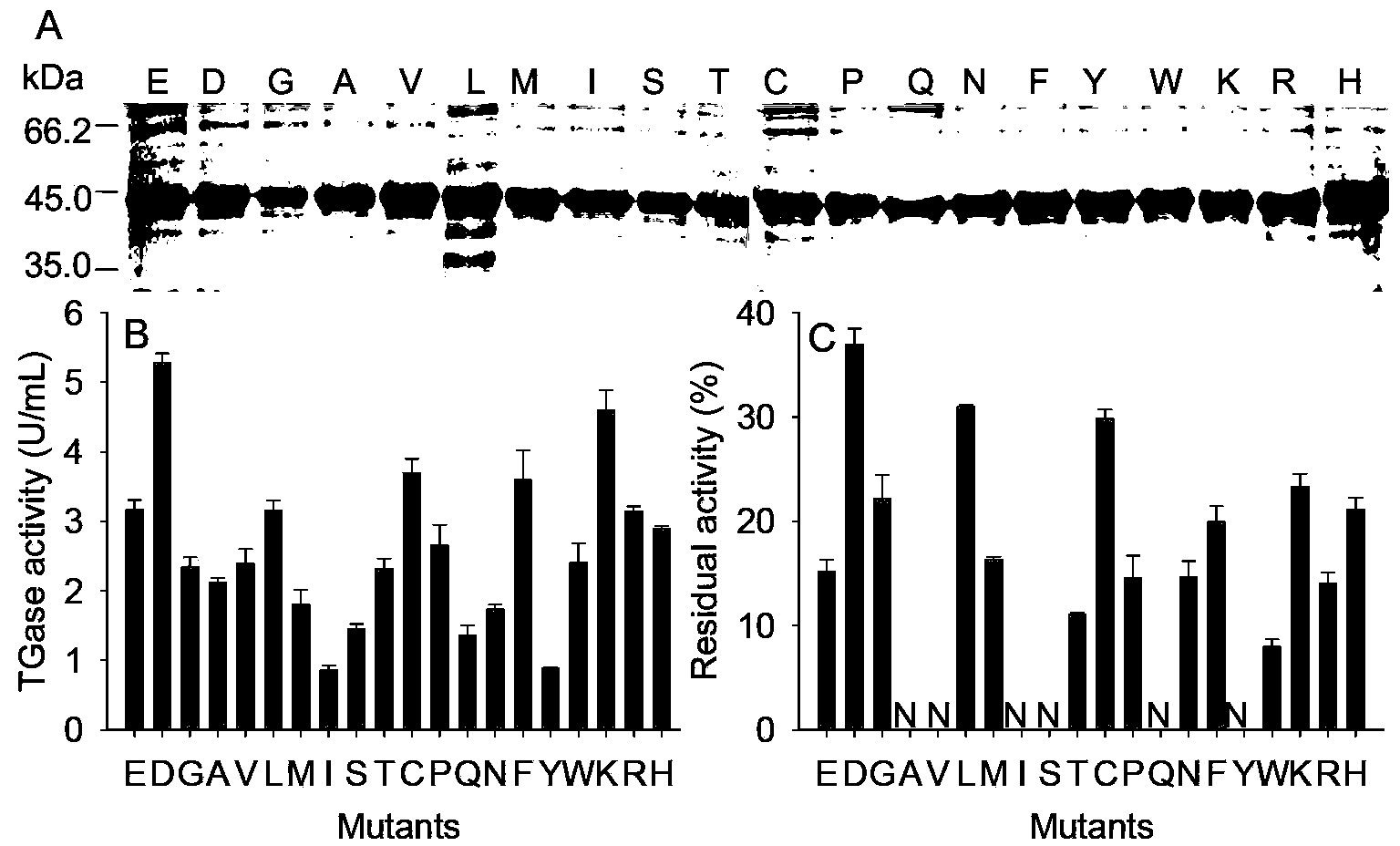
The invention provides glutamine transaminase with improved enzymatic activity, wherein the amino acid sequence is shown as SEQ ID NO.1. According to the invention, amino acid at the N end of MTG (Microbial Transglutaminase) maturase is subjected to deficiency and saturation mutation by taking the efficient expression of MTG in colibacillus as an improvement platform, and a mutant strain with good enzymatic property is obtained. The enzyme activity is improved by 1.85 times, and the thermal stability is improved by 2.7 times. The modified enzyme is more suitable for industrial applications, the production cost can be lowered, and the production efficiency can be improved.
Owner:JIANGNAN UNIV
Double-bioenzyme modified blending biological material containing gelatin and chitosan and preparation method and application thereof
InactiveCN102824654BImprove stabilityImprove mechanical propertiesAbsorbent padsProsthesisGlutaminaseTyrosinase
The invention provides a double-bioenzyme modified blending biological material containing gelatin and chitosan and a preparation method and application of the double-bioenzyme modified blending biological material. The blending biological material comprises gelatin and chitosan which are used a blending main body and microbial transglutaminase and tyrosinase which are used as a modifier. The stability of the blending biological material is enhanced, and the mechanical property thereof is improved; furthermore, the material has antimicrobial activity, and has good application prospect and excessively wide application in the fields of biomedical materials and a biomedicine, such as the reduction of infection and inflammatory response, antibiosis, wound protection, a biologically degradable skin dressing, a wound healing agent, artificial skin, and the like; and meanwhile, the material is simple in preparation technology, easy to control, free from side and toxic effects and temperate in preparation condition.
Owner:WUHAN UNIV OF TECH
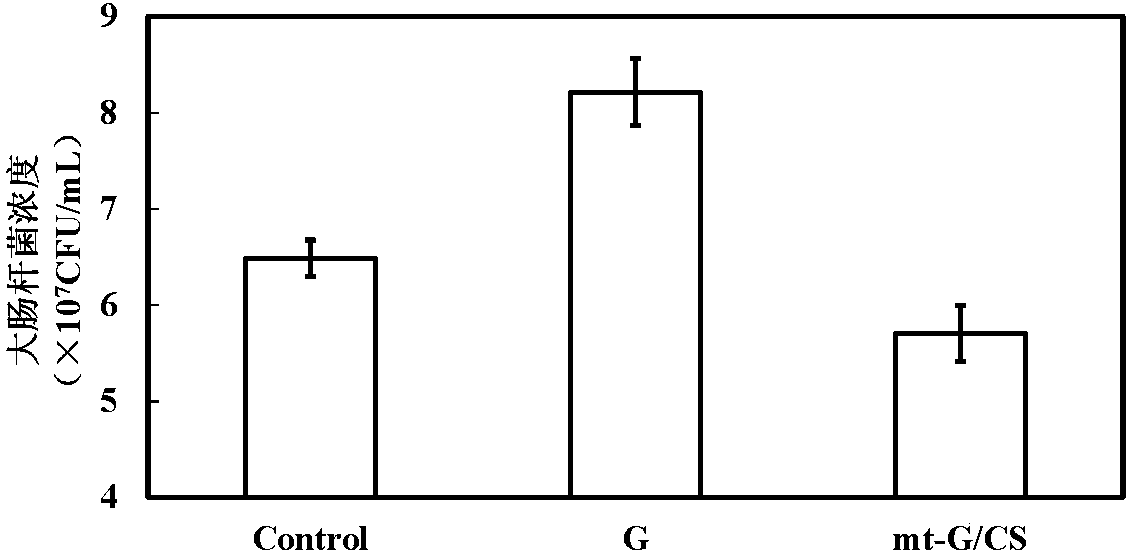
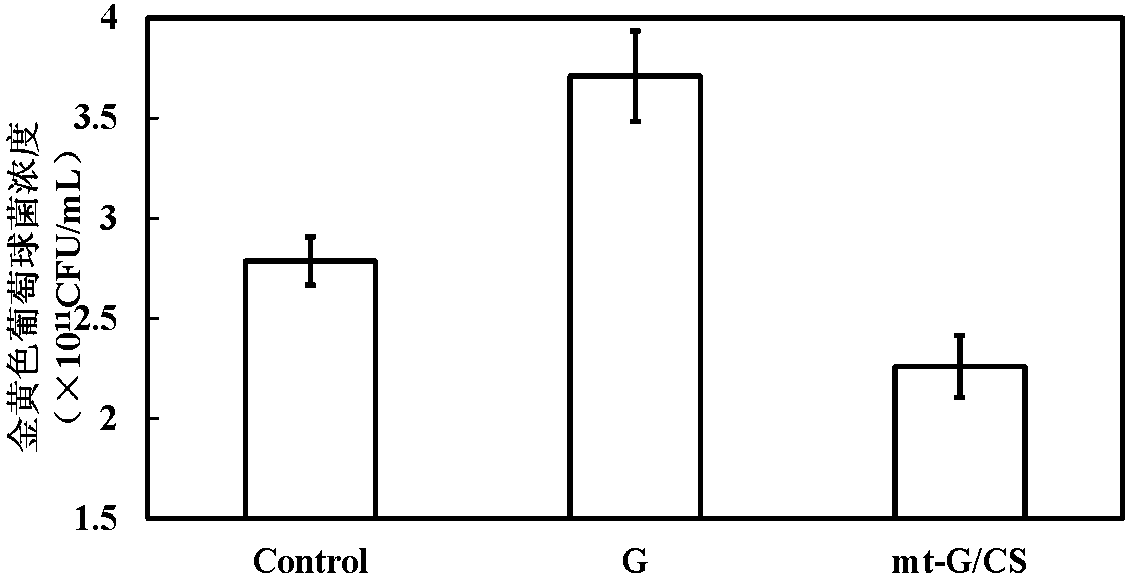
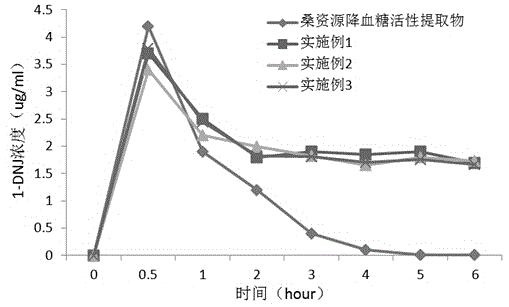
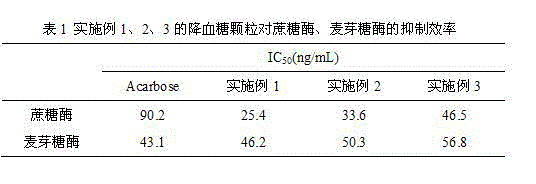
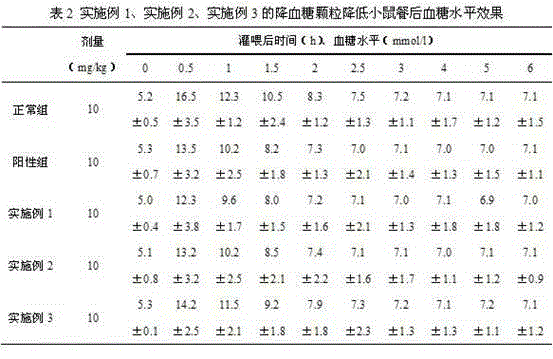
Mulberry resource long-acting hypoglycemic medicine composition as well as granules and preparation method thereof
InactiveCN104474026AReduce releaseReduce absorptionMetabolism disorderGranular deliveryBiotechnologyMicrobial transglutaminase
The invention discloses a mulberry resource long-acting hypoglycemic medicine composition as well as granules and a preparation method thereof. The mulberry resource long-acting hypoglycemic medicine composition comprises a mulberry resource hypoglycemic activity extract, a pueraria root flavonoid extract, gelatin and microbial transglutaminase; and the preparation method of the mulberry resource long-acting hypoglycemic granules comprises the following steps: preparing the mulberry resource hypoglycemic activity extract, preparing the pueraria root flavonoid extract, preparing the hypoglycemic granules, and the like. The mulberry resource hypoglycemic medicine composition and the granules prepared by using the method disclosed by the invention can be used for controlling the level of postprandial blood sugar in multi-way and long-acting modes.
Owner:SOUTHWEST UNIVERSITY
Microbial transglutaminase and daily bleeding stopping products prepared thereby
ActiveCN103820411AImprove recovery effectGood hemostasisPeptide/protein ingredientsCatheterBiotechnologySide effect
The invention discloses microbial transglutaminase and rapid bleeding stopping products for traumatic blood loss in daily life. The products adopt mutant microbial transglutaminase as a main active substrate or an effective component. The raw material microbial transglutaminase used in the invention is a mutant enzyme obtained through gene mutation in the circumstance of not changing the functions and the original low toxicity of traditional microbial transglutaminase, wherein compared with wild type enzymes, the mutant enzyme has amino acid mutagenesis at 4 loci. The enzyme has the advantages of excellent heat resistance and good stability at normal temperature. The rapid bleeding stopping products for the traumatic blood loss in daily life, which are prepared through the mutant enzyme, comprise patches, creams (ointments and plasters), pastes and liniments. Proved by tests, the products disclosed by the invention have the effects of rapidly stopping bleeding, easing pain, preventing wound adhesion, blocking bacteria and the like and have the advantages of low toxic or side effects and convenience in use.
Owner:李肯
Purification method of MTGase (Microbial Transglutaminase) crude enzyme
ActiveCN107058255AAchieve purificationSimple processAcyltransferasesPurification methodsMicrobial transglutaminase
The invention discloses a purification method of an MTGase (Microbial Transglutaminase) crude enzyme. The purification method comprises the following steps: dissolving MTGase crude enzyme powder with water to prepare an MTGase crude enzyme solution with the concentration of 1 weight percent to 2 weight percent; preparing a PEG (Polyethylene Glycol) / (NH4)2SO4 double-water-phase solution to form an upper-layer PEG phase and a lower-layer salt phase; uniformly mixing the MTGase crude enzyme solution with the PEG / (NH4)2SO4 double-water-phase solution; standing at 20 DEG C to 40 DEG C for 1h to 2h; enabling MTGase to enter the salt phase in a double-water-phase system and collecting the salt phase in the double-water-phase system; carrying out nanofiltration membrane treatment on the collected salt phase and collecting inter-membrane liquid of the nanofiltration membrane treatment, so as to obtain purified MTGase. The purification method has the advantages that purification is simple and convenient, the purity of the MTGase obtained by purification and separation is relatively high and the recycling rate is high.
Owner:JIMEI UNIV
Method for improving stability of gliadin granules
The invention relates to a method for improving the stability of gliadin granules. Specifically, the invention provides a preparation method of microbial transglutaminase (MTGase) catalyzed cross-linked sodium caseinate, and the method comprises the step: carrying out an MTGase catalyzed cross-linking reaction by taking sodium caseinate as a substrate and MTGase as a catalyst to obtain the MTGase catalyzed cross-linked sodium caseinate. The gliadin-cross linked sodium caseinate composite nanogranules formed by the MTGase catalyzed cross-linked sodium caseinate and the gliadin disclosed by the invention can be used as a delivery carrier of food active ingredients, have the characteristic of slow release, and can improve the solubility, stability, bioaccessibility and biological activity of the food active ingredients.
Owner:SHANGHAI JIAO TONG UNIV
C-terminal lysine conjugated immunoglobulins
ActiveCN108602878AIn-vivo radioactive preparationsImmunoglobulinsMicrobial transglutaminaseIntravenous gammaglobulin
Provided herein are conjugated immunoglobulins and methods for generating conjugated immunoglobulins using a microbial transglutaminase.
Owner:EISIA R&D MANAGEMENT CO LTD
A rapid hemostasis product for war wounds and its preparation method
ActiveCN103816562BImprove recovery effectImprove heat resistancePeptide/protein ingredientsCatheterBiotechnologyDisease
Owner:EAST CHINA NORMAL UNIV
A protein-grafted natural polysaccharide and its preparation method and application
The invention discloses protein-grafted natural polysaccharide, a preparation method and an application thereof, and concretely relates to bio-protein-grafted chitosan oligosaccharide, a preparation method and an application, which belongs to the high-molecular modification field. The bio-protein-grafted chitosan oligosaccharide is obtained by dissolved chitosan oligosaccharide and a bio-protein solution under effect of a catalyst microbial transglutaminase. According to the invention, reaction condition is mild, technology is simple, and the reaction substances are natural macromolecule with characteristics of environmental protection. The prepared water-soluble chitosan oligosaccharide bio-protein copolymer has good film forming ability, moisture-absorption effect, moisture-retention effect and antioxidation effect.
Owner:湖南尔康明胶有限公司
Transglutamine tag for efficient site-specific bioconjugation
ActiveUS11130818B2Easy to useImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical non-active ingredientsAntiendomysial antibodiesMicrobial transglutaminase
The present invention provides novel peptide sequences for use in microbial transgluatminase-mediated, in particular mTG2-mediated bioconjugations, in particular for the manufacture of antibody-drug-conjugates. Further disclosed are bioconjugation methods employing mTG2 and the novel peptide sequence motifs of the invention. The present invention further provides proteins comprising the novel sequence motifs of the invention as well as polynucleotides encoding the same.
Owner:MERCK PATENT GMBH
Method for improving specific activity of microbial transglutaminase
InactiveCN103421755AIncreased specific enzyme activitySuitable for industrial applicationsBacteriaTransferasesStreptomyces hygroscopicusMicrobial transglutaminase
The invention discloses a method for improving specific activity of microbial transglutaminase and belongs to the field of enzyme engineering. Mutant enzyme with specific activity improved by 40 percent is obtained by using a site-specific mutagenesis technology to insert a loop, coming from the interior of the transglutaminase maturase and playing an importance effect to TGase catalytic activity, or an amino acid sequence, coming from the vicinity of an catalytic activity center, between streptomyces hygroscopicus transglutaminase leading peptide and maturase, and the method is more suitable for industrial production and application.
Owner:JIANGNAN UNIV
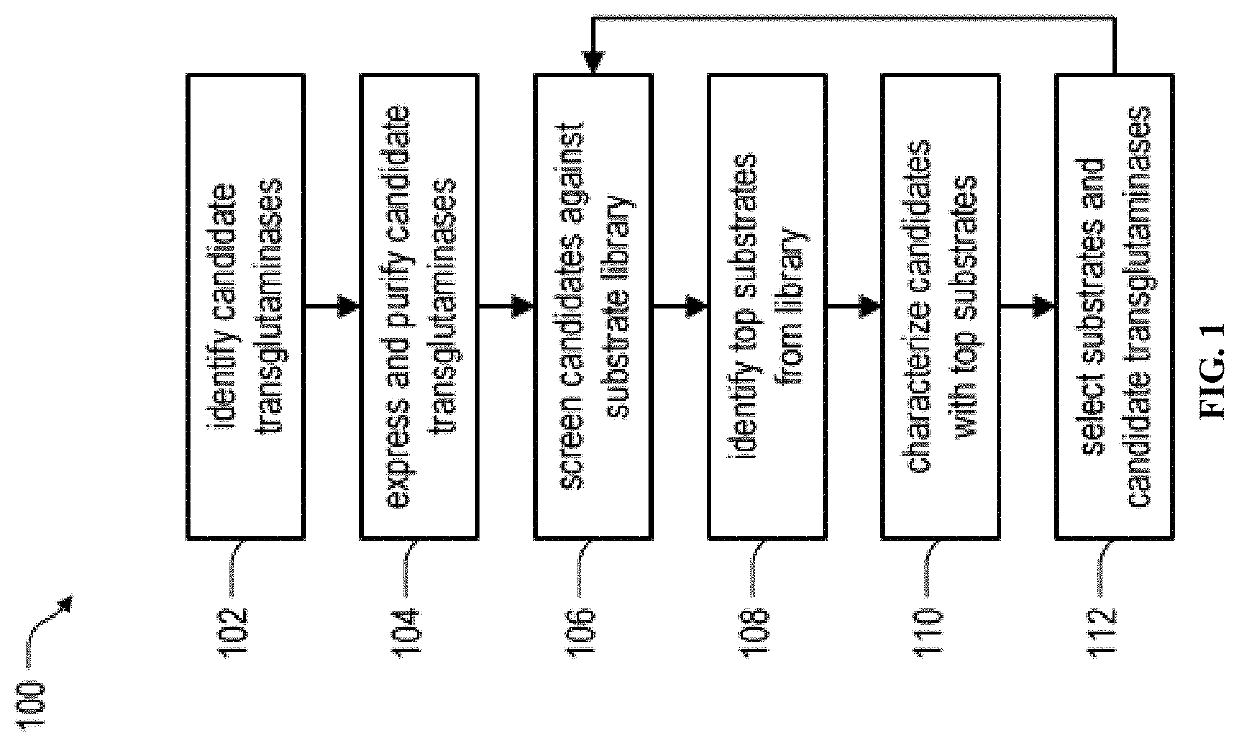
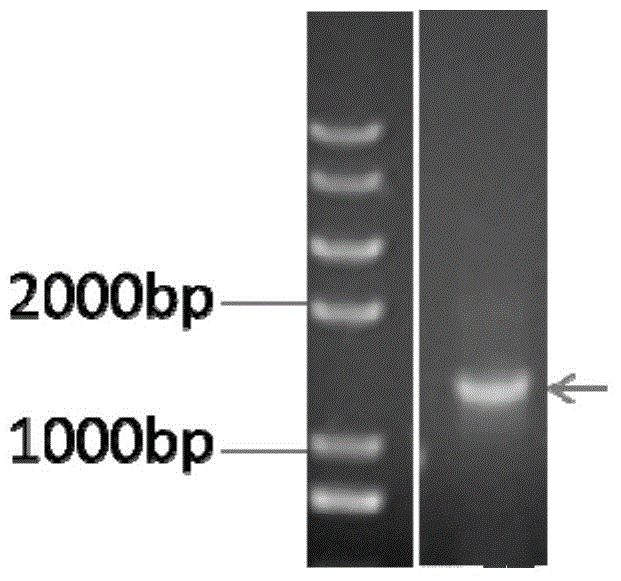
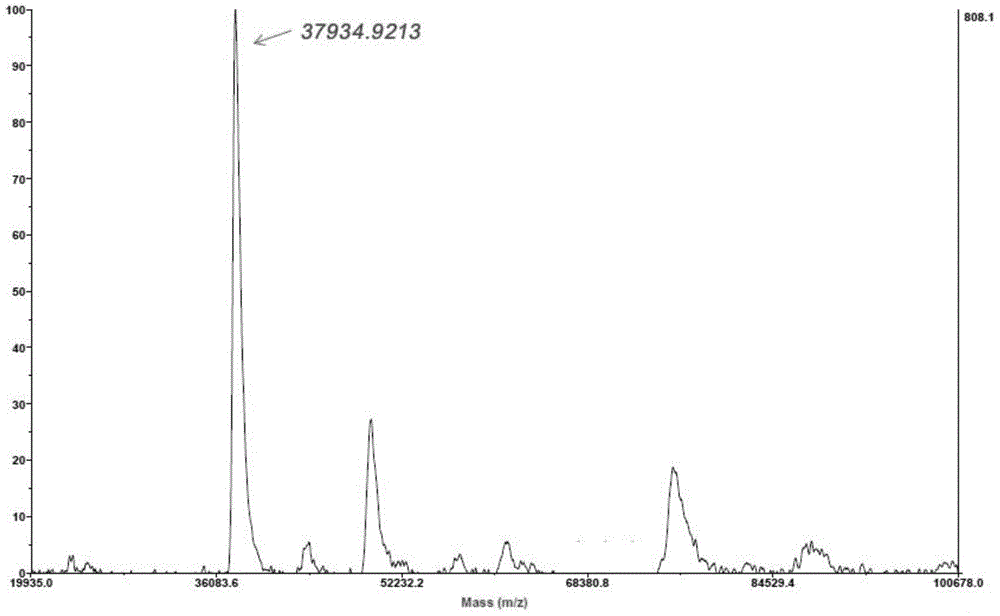
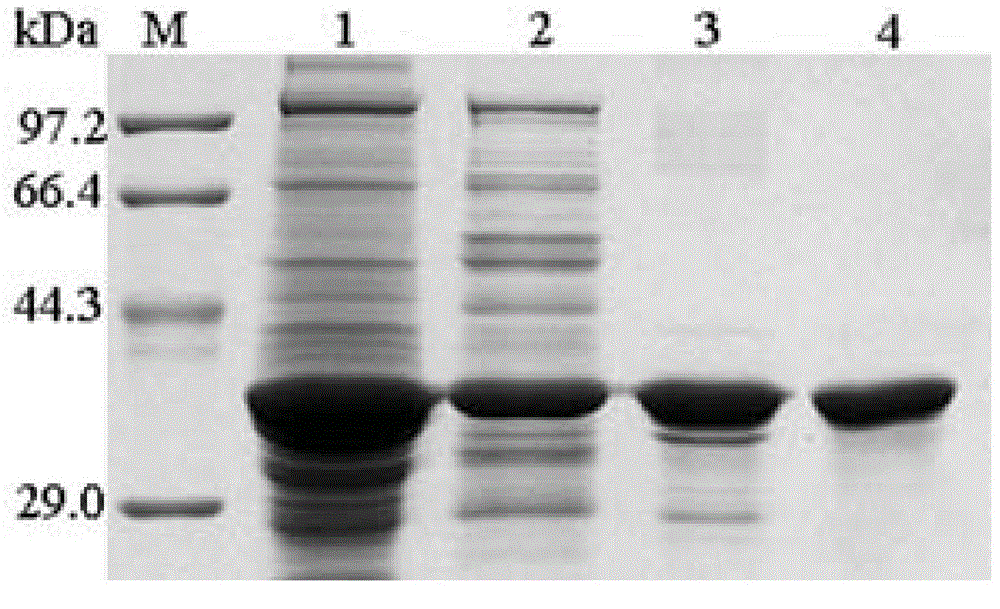
System and method for identification and characterization of transglutaminase species
ActiveUS20200249231A1Antibody mimetics/scaffoldsBiological material analysisMicrobial transglutaminaseMicrobacterium
In one aspect, the present disclosure provides a system and method for the identification and characterization of a transglutaminase. Further, the present disclosure provides transglutaminase enzymes for forming isopeptide bonds, methods of forming isopeptide bonds in the presence of transglutaminases, and substrate tags for use with transglutaminases. In another aspect, the present disclosure provides glutamine-containing substrates (or Q-tag substrates) that are more resistant to proteases / clipping and therefore, more stable, than other Q-tag substrates, and their uses in substrate tags for cross-linking to an amine-donor tag via an isopeptide bond mediated by a microbial transglutaminase.
Owner:ROCHE SEQUENCING SOLUTIONS INC
Rapid hemostatic product for clinical operation wounds, as well as preparation method and application of rapid hemostatic product
ActiveCN103820412ASignificant metabolic disturbanceImprove heat resistancePeptide/protein ingredientsCatheterDiseaseBiotechnology
The invention discloses a rapid hemostatic product for clinical operation wounds, as well as a preparation method and application of the rapid hemostatic product. The rapid hemostatic product comprises a matrix containing a microbial transglutaminase (mT6), wherein the amino acid sequence of the transglutaminase is the protein sequence coded by the gene shown in SEQ ID NO.: 3, or the protein sequence shown in SEQ ID NO.: 4. Specifically, the rapid hemostatic product is selected from a hemostatic, a hemostatic device, a hemostatic first-aid kit or a common hemostatic product; the matrix adopts a fluid or semisolid form, which can be absorbed by a human body, or a plastic gel or colloid form. The rapid hemostatic product contains the microbial transglutaminase and gelatin. The rapid hemostatic product has the advantages of rapid hemostatic property, convenience for use, prevention on organ adhesion, promotion on wound healing, low toxic and side effects, complete absorption and the like. The rapid hemostatic product can be used as a medical hemostatic material or product for clinical operation wounds, can stop bleeding rapidly, prevents organ adhesion, is easy to absorb, has little possibility of spreading diseases, and is low in cost.
Owner:李肯
Method for presymptomatic diagnosis of coeliac disease and gluten sensitivity
ActiveUS10571466B2Bacterial antigen ingredientsMicrobiological testing/measurementMicroorganismMicrobial transglutaminase
The invention relates to the use of an immunologically reactive microbial transglutaminase or its immunologically reactive parts or analogues, which are present in a complex with gliadin or its immunologically reactive parts or analogues, for the diagnosis and / or therapy control of coeliac disease or sprue as well as gluten sensitivity, and a kit for determining the diagnosis and / or therapy control of coeliac disease or sprue as well as of gluten sensitivity, by means of the previously mentioned complex.
Owner:AENEAS GMBH & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com