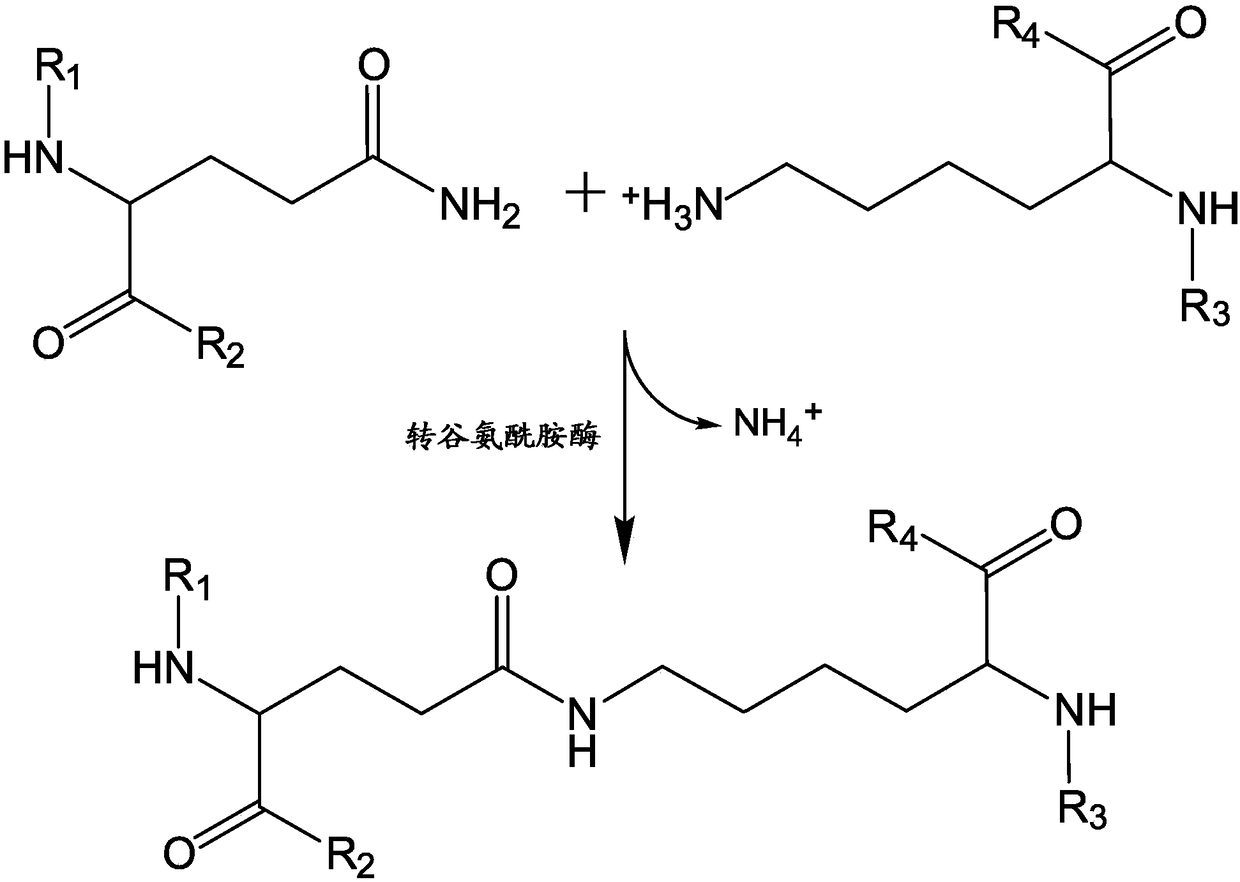
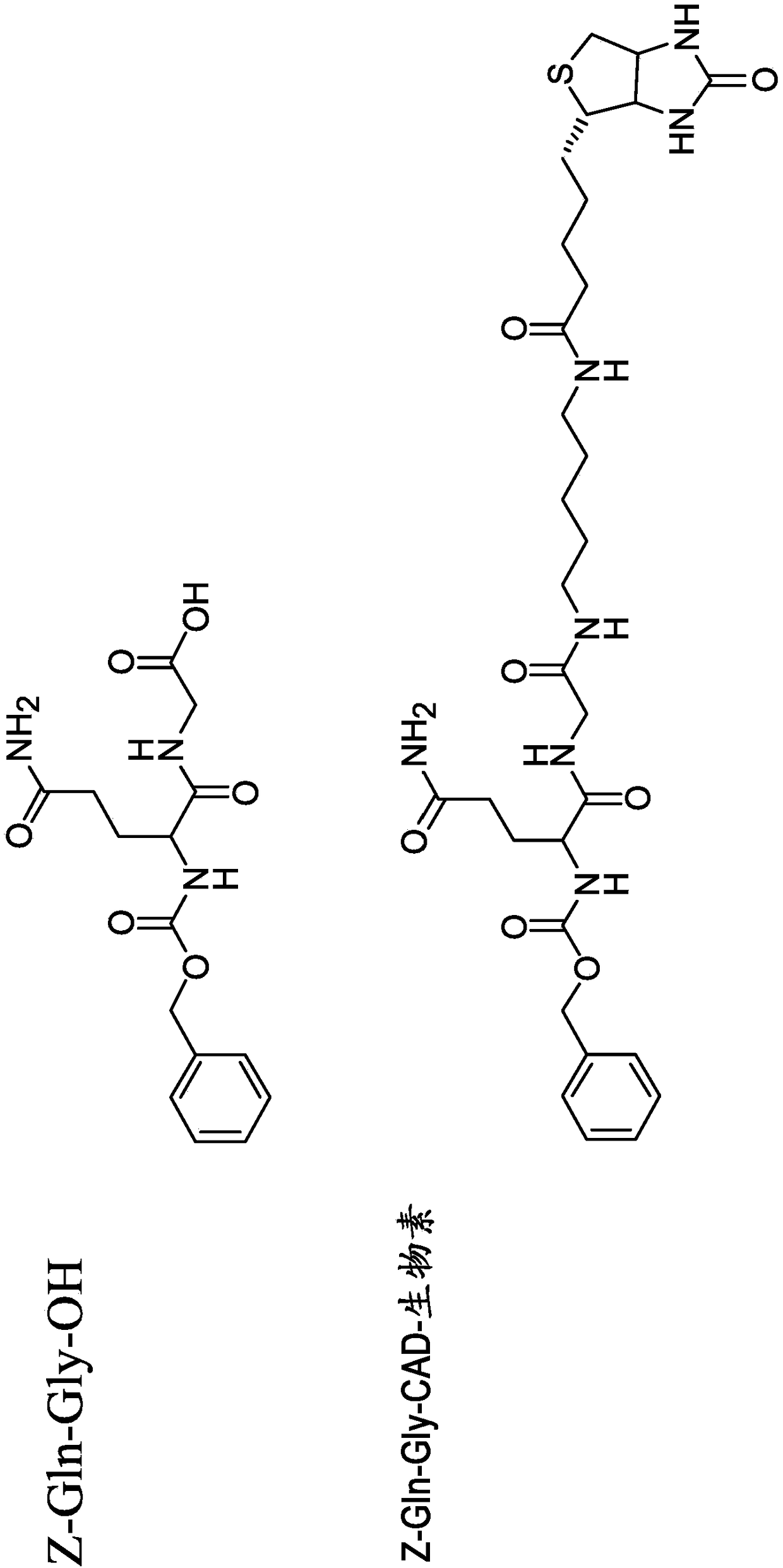
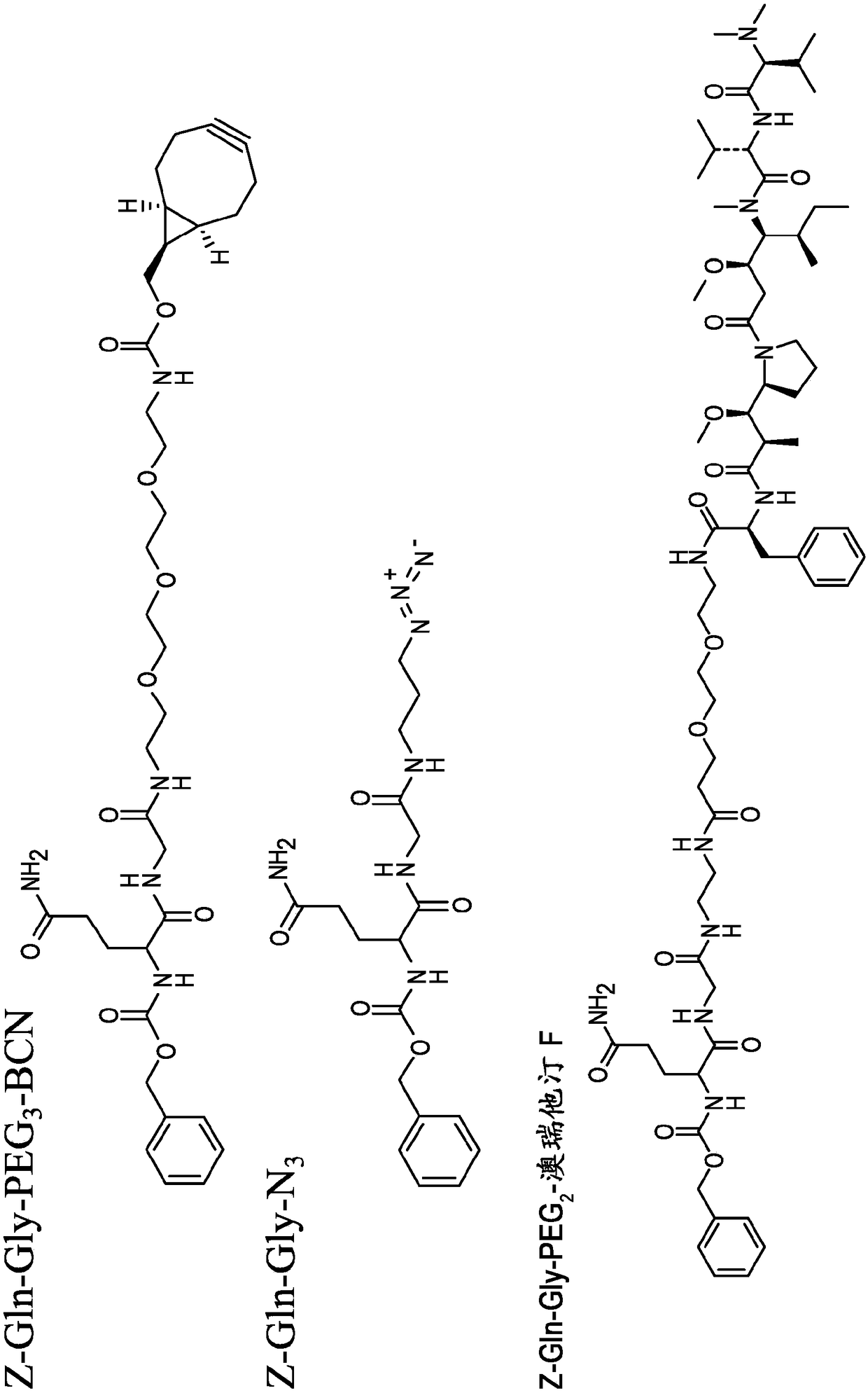
C-terminal lysine conjugated immunoglobulins
A technology of immunoglobulin and terminal lysine, applied in the field of C-terminal lysine conjugated immunoglobulin, which can solve the problem of low specificity of acyl acceptor amine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0216] Example 1: Materials and methods
[0217] mutagenesis
[0218] Mutations were generated using Stratagene's QuikChange XL according to the manufacturer's protocol. Desired mutations were confirmed by DNA sequencing.
[0219] Transfection and stable cell line generation
[0220] For each ml of cells to be transfected with ExpiFectamine, 333.3 ng of HC plasmid and 333.3 ng of LC plasmid were incubated in 50 μL of Opti-MEM (ThermoFisher) for 5-10 min. Similarly, 2.67 μL of ExpiFectamine was incubated in 50 μL of Opti-MEM. Add the ExpiFectamine solution to the DNA mixture and incubate at room temperature for 20-30 minutes. Add the DNA:ExpiFectamine mixture to the cells while rotating and incubating at 37 °C, 8% CO 2 Incubate with shaking at 125rpm. The next day, 5 µL of Enhancer 1 and 50 µL of Enhancer 2 per mL of cells were added to the transfection and the incubation continued for another 7-10 days.
[0221] 1 to 3 days after transfection, select for antibody-ex...
Embodiment 2
[0247] Example 2: Analysis of solvent exposed lysine on IgG antibodies
[0248] IgG was detected 1 -κFab (antibody 01,4F3F), IgG 1 -λFab(4HK0) and IgG 1 Crystal structure of Fc(1FC1) for potential acyl acceptor sites. Since microbial transglutaminases tend to prefer the solvent-exposed substrate glutamine and intracyclic lysine {Spolaore, 2012 17 / id}, solvents were highlighted using Discovery Studio v4.5 with a 1.4 angstrom probe radius Exposed lysine (Figure 4). In Antibody 01 VH there are 7 solvent exposed lysines and 3 are in the loop. Since the number of lysines can vary between mAbs utilizing different variable region families and somatic hypervariation, lysines in the VH regions of five other antibodies were also analyzed based on the similar positions of the residues in the 4F3F structure of solvent exposure. These VH regions may contain 1-5 solvent-exposed lysines, 1 or 2 present in loops. In antibody 01 VK there are 6 solvent exposed lysines and 4 are in the lo...
Embodiment 3
[0263] Example 3: A single amino acid extension is sufficient for transamidation of Lys447
[0264] Lys447 is normally cleaved by carboxypeptidase B during recombinant IgG expression in HEK293 and CHO cells {Harris, 1990 7 / id; Harris, 1995 6 / id; Dick, 2008 3 / id}. However, addition of KTag to the HC C-terminus blocks the removal of Lys447, thereby allowing microbial transglutaminases to utilize Lys447 as an acyl acceptor site. To determine whether microbial transglutaminases could use Lys447 as a KTag-free acyl acceptor, Lys447 was cleaved by adding one or two leucines to the C-terminus of Antibody 01 (Antibody 01-HC-L or Antibody 01-HC-L or Antibody 01-HC-LL) to block. Purified mAbs were incubated with Z-Gln-Gly-CAD-biotin and microbial transglutaminase and analyzed for the mass of deglycosylated HC. Indeed, addition of one or two leucines preserved Lys447 and modified the HC with a single acyl donor substrate consistent with transamidation of Lys447 (Figure 8; Table 3).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com