Glutamine transaminase with improved heat stability and application thereof
A technology of glutamine and transaminase, applied in the field of transglutaminase, can solve problems such as poor thermal stability, and achieve the effects of improving production efficiency and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Simulation of MTG crystal structure derived from Streptomyces hygroscopicus
[0020] Using the reported TGase crystal structure of S. mobaaensis as a template, the crystal structure of S. hygoscopicus TGase was simulated on the swiss-model website (http: / / swissmodel.expasy.org / ).
Embodiment 2
[0021] Example 2: Transglutaminase with Improved Thermostability
[0022] Based on the transglutaminase encoding gene published by Genbank: EU477523, an amino acid tag is added to its C-terminus, wherein the amino acid sequence of the tag is shown in SEQ ID NO.1 or SEQ ID NO.2, among which SEQ ID NO.1 is preferred .
Embodiment 3
[0023] Example 3: Obtaining mutant strains with improved thermostability (linker9, linker13)
[0024] 1. Use chemical total synthesis or PCR to clone the gene encoding transglutaminase Genbank: EU477523, add different types of tags that help improve thermal stability at the C-terminus of MTG, and clone the modified transglutaminase gene into carrier.
[0025] 2. Transform the plasmid with correct sequencing into E.coli BL 21, select the transformant and inoculate it into LB liquid medium, culture at 37°C for 12 hours, and transfer it to TB medium with an inoculation volume of 3%. Bacteria grow to OD 600 When it was 2, IPTG was added to induce, and the culture temperature was lowered to 20°C, and cultured for 48h.
[0026] 3. Collect the fermentation supernatant, detect the enzyme activity of the fermentation supernatant, and purify the sample with His-nickel column.
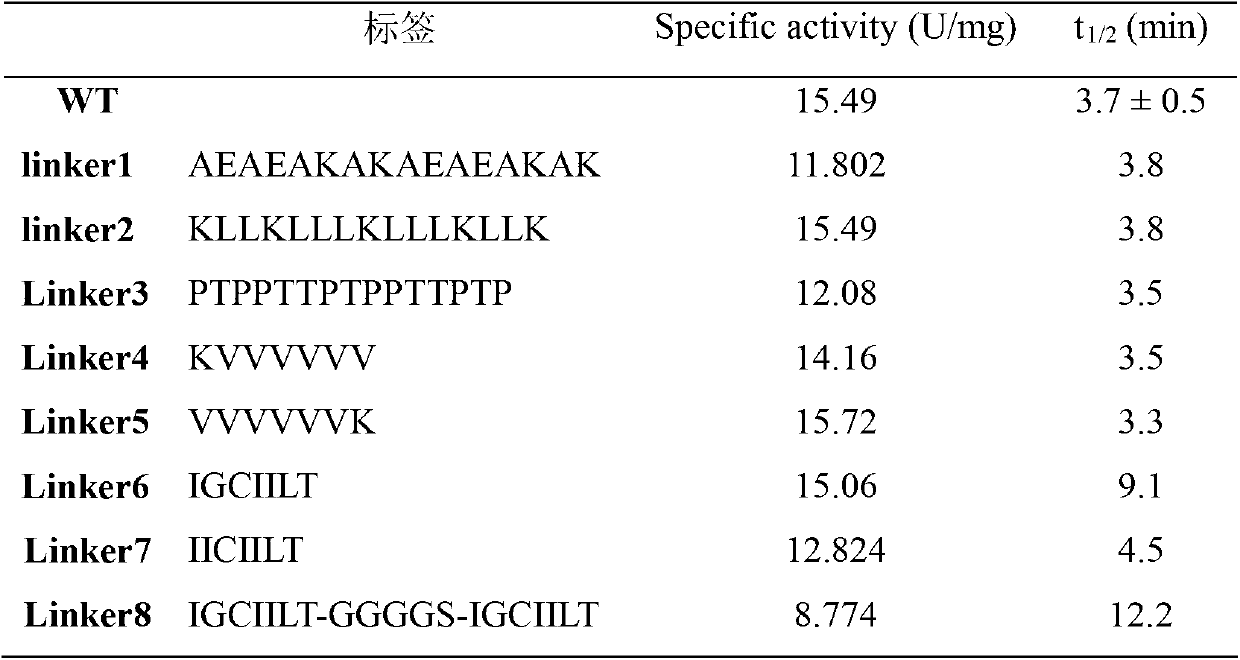
[0027] 4. The specific enzyme activity and Km value of the purified MTG were measured, and the results are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com