Nitrile hydratase mutant and application thereof
A technology of nitrile hydratase and mutants, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low tolerance, low end product acrylamide, inactivation of nitrile hydratase, etc., and achieve improved substrate tolerance, Good enzymatic properties and improved thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: Construction of nitrile hydratase mutant
[0080] Specific steps are as follows:
[0081] 1. (1) Construction of mutant βL48D:
[0082] Chemically synthesize the nitrile hydratase NHase gene (nucleotide sequence shown in SEQ ID NO.3), and clone the gene into the NdeI and EcoRI restriction sites of the pET24a plasmid, completed by Suzhou Jinweizhi, to obtain pET24a-NHase recombinant plasmid. Using pET24a-NHase as a template, use the primers shown in Table 1 to perform PCR under the conditions shown in Table 2 to obtain a PCR product, transform the obtained PCR product into E.coli JM109 competent cells and send it to Suzhou Jinweizhi for sequencing, and the sequencing result is correct The plasmid is the recombinant plasmid pET24a-βL48D carrying the mutant gene; transform the recombinant plasmids pET24a-βL48D and pET24a-NHase into E.coli BL21 strains for expression, and pick the transformants for verification. The correct verification is: recombination St...
Embodiment 2
[0094] Example 2: Nitrile hydratase mutant half-life
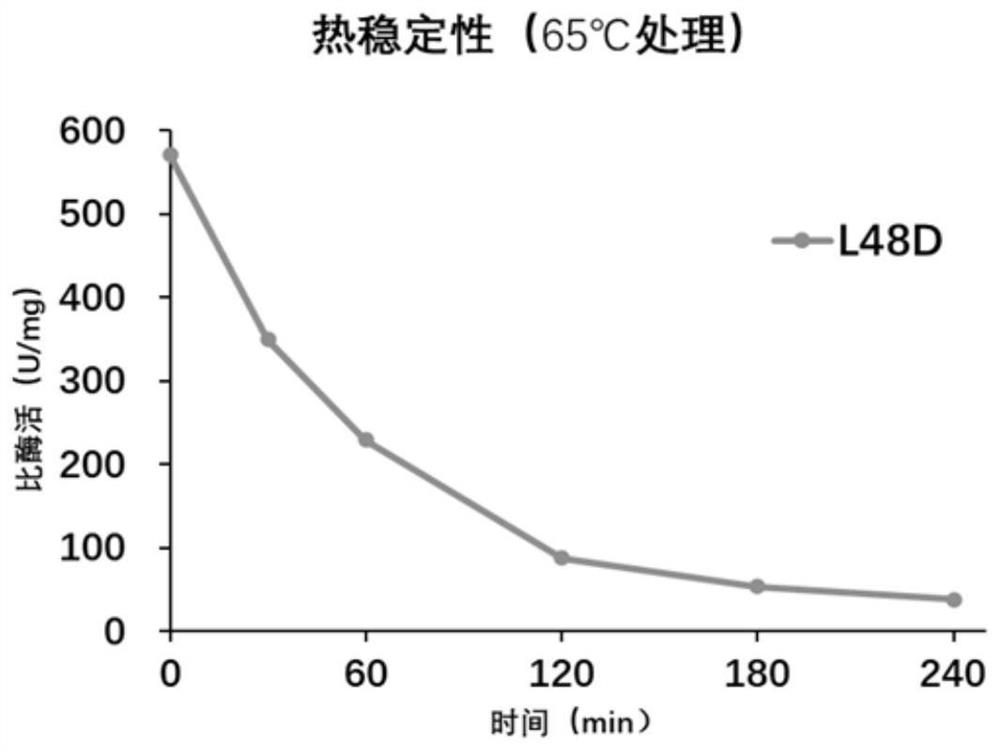
[0095] Nitrile hydratase still has good thermal stability after mutation. In this embodiment, the mutant βL48D is taken as an example to measure its half-life:
[0096] 10 μL of 0.5 mg / ml mutant enzyme purified in Example 1 was added to 500 μl of buffered reaction system, treated in a 65°C metal bath for 0 min, 30 min, 60 min, 120 min, 180 min, and 240 min, and the residual enzyme activity was determined.
[0097] Such as figure 1 As shown, it was found that the half-life of the mutant enzyme βL48D was 43min at 65°C.
[0098] The results showed that the improved enzyme activity still had good thermal stability.
Embodiment 3
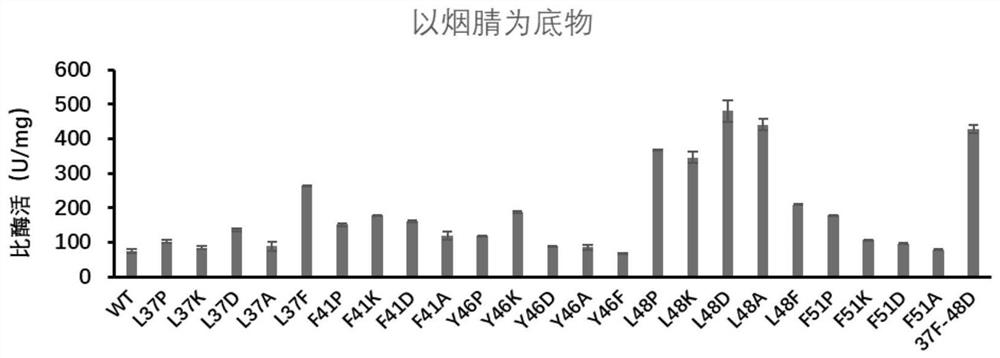
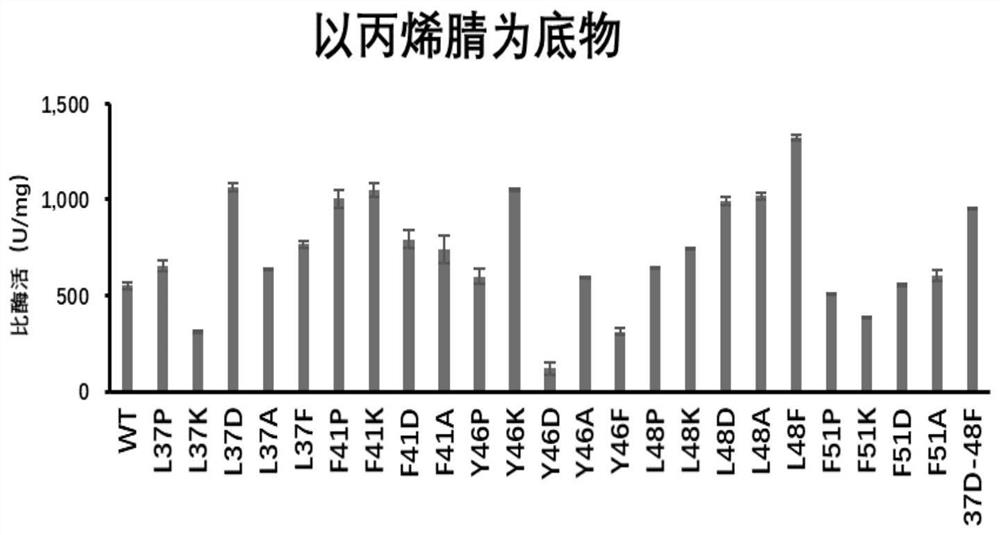
[0099] Embodiment 3: Nitrile hydratase is assayed to substrate nicotinyl enzyme activity
[0100] Add 0.5 mg / ml wild enzyme and mutant pure enzyme solution 10 μL respectively to 490 μL 200 mM nicotinonitrile solution obtained in Example 2 to obtain a reaction system. Acetonitrile terminates the reaction to obtain a reaction liquid, and the supernatant of the reaction liquid is taken and passed through a 0.22 μm membrane as a sample for liquid phase determination, and the specific enzyme activity of nitrile hydratase is detected. The result of the reaction is as figure 2 and as shown in Table 4:
[0101] Table 4: Specific activity of different nitrile hydratase mutants on the substrate nicotinonitrile
[0102] sample name Specific enzyme activity (U / mg) WT 73.49 L37P 101.95 L37K 84.62 L37D 135.42 L37A 88.73 L37F 265.68 F41P 150.19 F41K 178.60 F41D 161.44 F41A 119.42 Y46P 117.70 Y46K 187.11 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com