Synthetic method for 2-(2-hydroxyphenyl)-2-oxyacetate
A technology of oxyacetate and hydroxyphenyl, which is applied in the field of synthesis of 2--2-oxyacetate, can solve the problems of multiple wastes, difficult separation, difficult control, etc., and achieve simple treatment, easy manipulation, and good reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
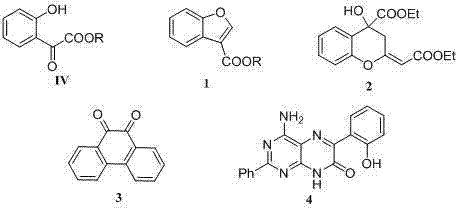
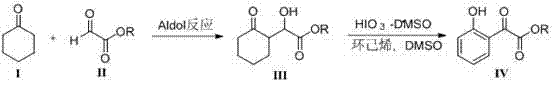
[0032] 2-Hydroxy-2-(2'-oxycyclohexyl) ethyl acetate ( III-1 )Synthesis
[0033] Add 1.0 g (4.9 mmol) ethyl glyoxylate ( II-1 , the commercially available quality fraction is 50% II-1 Toluene solution) and 4.8g (49mmol) cyclohexanone ( I ), slowly dropwise added 29 mg (0.25 mmol) of trifluoroacetic acid, stirred at room temperature, concentrated under reduced pressure after 24 h, and the residue was purified by silica gel column chromatography (eluent: the volume ratio of petroleum ether and ethyl acetate was 2:1 ), to get 0.64g product 2-hydroxyl-2-(2'-oxycyclohexanone) ethyl acetate ( III-1 ), light yellow oily liquid, yield 65.4%.
[0034] 1 H NMR (300MHz, CDCl 3 ) δ:4.68(d, J =2.4Hz, 1H), 4.26(q, J =7.1Hz, 2H), 1.87-2.98(m, 10H), 1.29(t, J =7.1Hz, 3H).
Embodiment 2
[0036] 2-(2-Hydroxyphenyl)-2-oxoacetic acid ethyl ester ( IV-1 )Synthesis
[0037] Get 2-hydroxyl-2-(2'-oxycyclohexyl) ethyl acetate ( III-1 ) 200mg (1.0mmol) in a sealed tube, add HIO 3 -DMSO 2.0mL, DMSO 1.0mL, cyclohexene 0.25mL, the reaction device was wrapped with tin foil, stirred at 45°C under temperature control, and monitored by TLC. After 12h, water (10mL) was added to stop the reaction; followed by extraction with ethyl acetate (10mL× 3), combined the ethyl acetate layers, dried over anhydrous sodium sulfate, filtered, and the concentrated residue was purified by column chromatography (eluent: the volume ratio of petroleum ether and ethyl acetate was 10:1); to obtain 116mg Product 2-(2-hydroxyphenyl)-2-oxoacetic acid ethyl ester ( IV-1 ), yellow oily liquid, yield 60.0%.
[0038] 1 H NMR (300MHz, CDCl 3 ) δ: 11.20(s, 1H), 7.70(dd, J 1 =1.5Hz, J 2 =8.0Hz, 1H), 7.61-7.55(m, 1H), 7.04(d, J =8.0Hz, 1H), 7.00-6.94(m, 1H), 4.49(q, J =7.1Hz, 2H), 1.44(t, J ...
Embodiment 3
[0039] Example 3 : Regarding the change in the preparation of ethyl 2-hydroxy-2-(2'-oxocyclohexyl)acetate ( III-1 ) Experimental research on the amount of trifluoroacetic acid in the process
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com