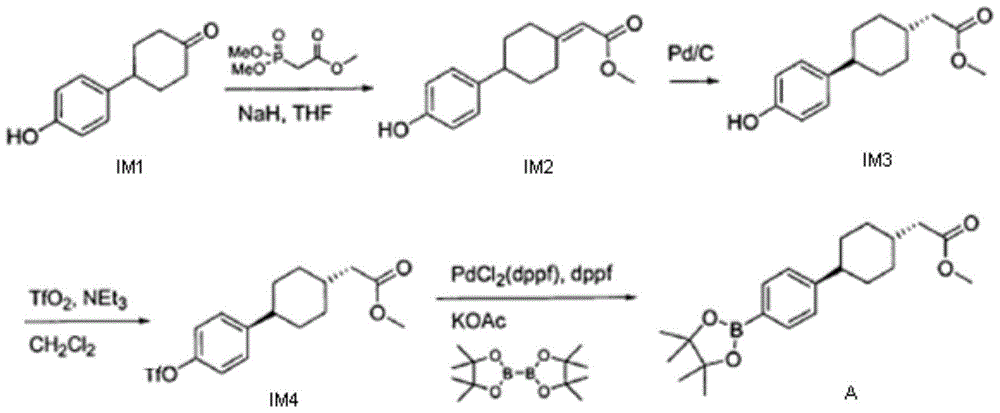
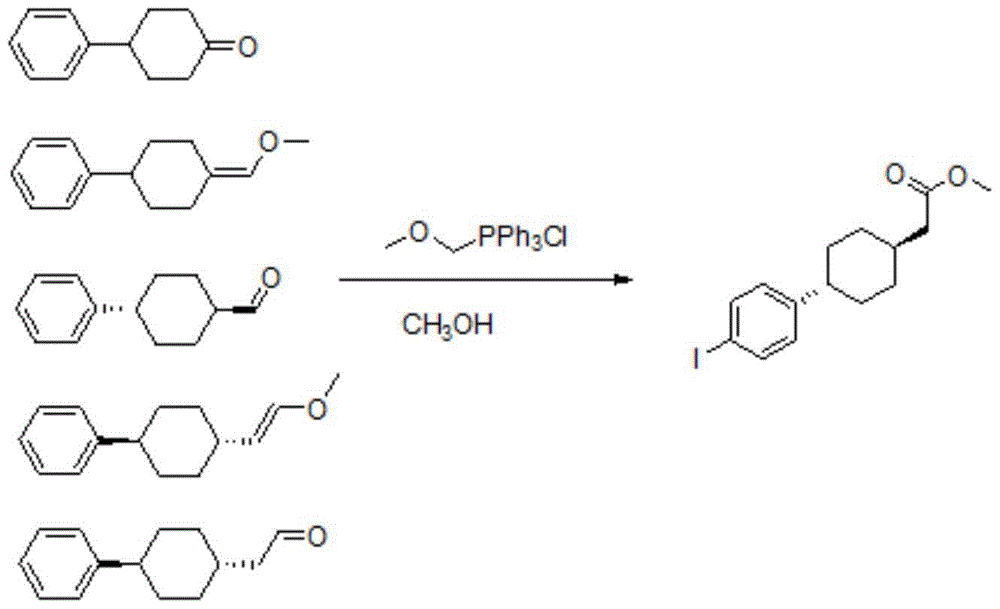
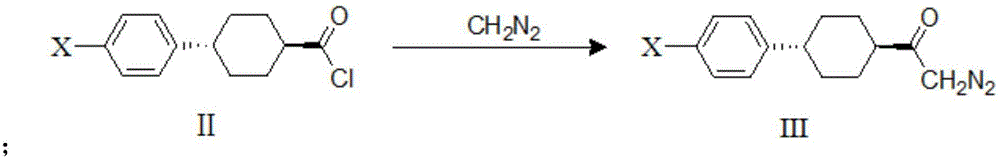A kind of preparation method of dgat-1 inhibitor intermediate
A technology of reagents and reaction solvents, applied in the field of preparation of pharmaceutical intermediates, can solve problems such as high raw material costs, many reaction steps, and instability, and achieve the effects of simplifying carbon chains, reducing raw material costs, and increasing total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Synthesis of trans-4-(4-chlorophenyl)cyclohexylformyl chloride:
[0036]
[0037] Add 28.7g of trans-4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid into a 250ml four-necked flask, then add 103.9g of toluene and 0.72g of N,N-dimethylformamide, and raise the temperature under nitrogen protection to 50°C. Shake 15.0 g of thionyl chloride and 19.9 g of toluene to form a uniform solution, control the temperature at 50° C., and slowly drop the above thionyl chloride solution into the reaction system. After dropping, the temperature was controlled and stirred at 50°C for 4 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and 247.7 g of toluene was added under nitrogen protection, and shaken to form a toluene solution of trans-4-(4-chlorophenyl)cyclohexylformyl chloride. It is a light yellow liquid, containing a small amount of white solid. This solution is directly used in the step of trans-4-(4-chlorophenyl)cyclohexyldiazone, ...
Embodiment 2
[0048] 1. Synthesis of trans-4-(4-bromophenyl)cyclohexylformyl chloride:
[0049]
[0050]Add 34g of trans-4-(4-bromophenyl)cyclohexyl-1-carboxylic acid into a 250ml four-necked flask, then add 105g of toluene and 0.75g of N,N-dimethylformamide, and heat up to 50 ℃. Shake 15.0 g of thionyl chloride and 20.5 g of toluene to form a uniform solution, control the temperature at 50° C., and slowly drop the above thionyl chloride solution into the reaction system. After dropping, the temperature was controlled and stirred at 50°C for 4 hours. After the reaction, the solvent was evaporated under reduced pressure, and 251.6 g of toluene was added under nitrogen protection, and shaken to form a toluene solution of trans-4-(4-bromophenyl)cyclohexylformyl chloride. It is a light yellow liquid, containing a small amount of white solid. This solution is directly used in the step of trans-4-(4-bromophenyl)cyclohexyldiazone, and the yield is quantitative.
[0051] 2. Synthesis of trans-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com