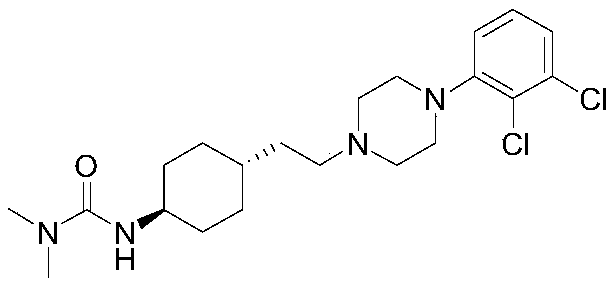
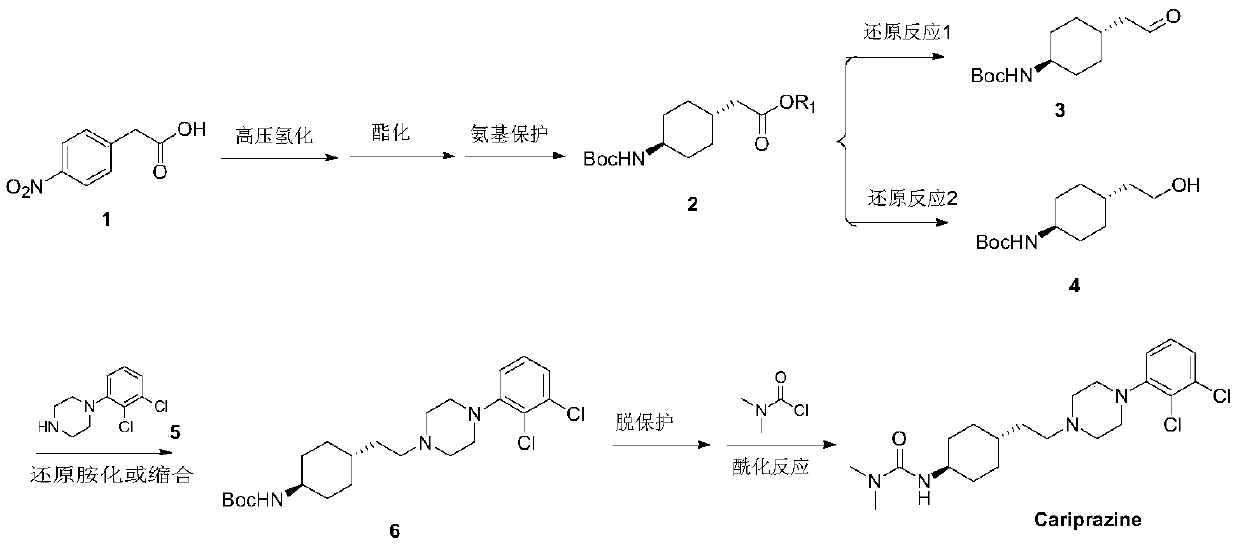
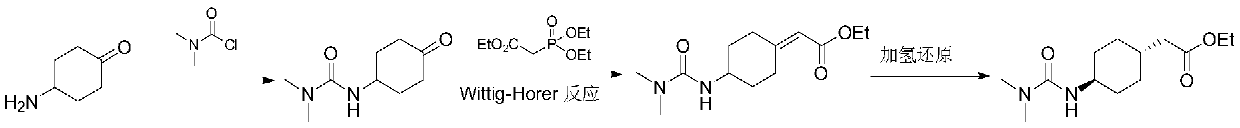
A kind of method of synthesizing cariprazine
A technology of piperazine and dichlorophenyl, applied in the field of synthesizing cariprazine, can solve the problems of long total reaction steps, expensive reagents and equipment requirements, etc., and achieve the effect of increasing the total yield and shortening the process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexyl)- Synthesis of 1,1-dimethylurea:
[0035]
[0036] Suspend trans-2-(4-(3,3-dimethylureido)cyclohexyl)acetic acid (105g, 0.46mol) in dichloromethane (500ml), slowly add thionyl chloride (82.1 g, 0.69mol), after addition, the system was heated to reflux and stirred for 4h. The reaction solution was concentrated to dryness under reduced pressure, and 300 ml of dichloromethane was added to obtain an acid chloride solution, which was set aside.
[0037] In another reaction flask was added 1-(2,3-dichlorophenyl)piperazine hydrochloride (135.4g, 0.51mol), triethylamine (116.2g, 1.15mol) and dichloromethane (1.3L) , cooled in an ice-water bath. Control the temperature < 15°C and add the prepared acid chloride solution dropwise. After the addition is complete, react at room temperature for 2 hours. After the reaction was completed, the organic phase was poured into water (1 L), separa...
Embodiment 2
[0039] Example 2: 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexyl)- Synthesis of 1,1-dimethylurea:
[0040]
[0041] Suspend trans-2-(4-(3,3-dimethylureido)cyclohexyl)acetic acid (10g, 43.8mmol) in dichloromethane (80ml), add triethylamine (6.6g, 65.7mmol ), the reaction solution was cooled to <-15°C, and isobutyl chloroformate (6g, 44mmol) was slowly added. After the addition, the temperature was controlled at -15~-10°C and stirred for 1 h to obtain a mixed anhydride solution, which was set aside.
[0042] Add 1-(2,3-dichlorophenyl)piperazine hydrochloride (12.9g, 48.2mmol), triethylamine (5.3g, 53mmol) and dichloromethane (60ml) in another reaction flask, stir Uniformly and slowly drop into the prepared mixed anhydride solution, temperature control <5°C. After the addition was completed, the mixture was naturally warmed to room temperature and stirred for 2h. After the reaction was completed, the reaction solution was quenched by adding 1...
Embodiment 3
[0043] Example 3: 3-(trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-2-oxo-ethyl}-cyclohexyl)- Synthesis of 1,1-dimethylurea:
[0044]
[0045] Suspend trans-2-(4-(3,3-dimethylureido)cyclohexyl)acetic acid (10g, 43.8mmol) in dichloromethane (80ml), add triethylamine (6.6g, 65.7mmol ), the reaction solution was cooled to <-5°C, and carbonyldiimidazole (8.5 g, 52.6 mmol) was added in batches. After the addition, the temperature of the reaction solution was raised to 10-15°C and stirred for 1-2 hours, and 1-(2,3-dichlorophenyl)piperazine hydrochloride (12.9 g, 48.2 mmol) was added in batches, and the temperature was controlled to <15°C. After the addition was completed, the mixture was naturally warmed to room temperature and stirred for 2h. After the reaction was completed, the reaction solution was quenched by adding 100 ml of water, adjusted to acidity with 6N hydrochloric acid solution, and the organic phase was separated. The organic phase was concentrated to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com