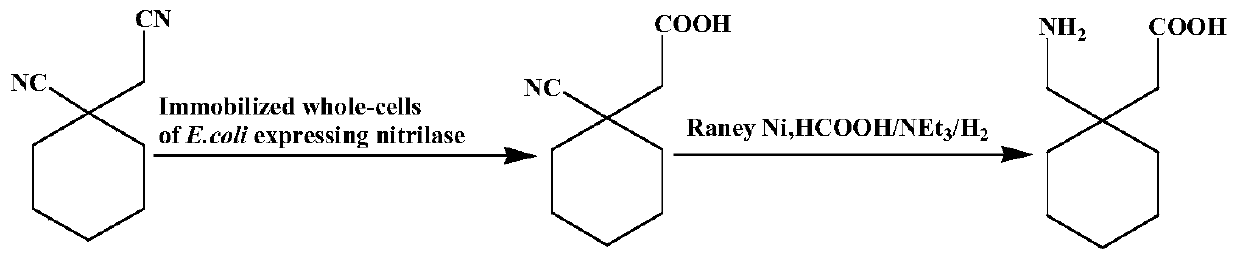
Utilize the method for directly synthesizing gabapentin with 1-cyano cyclohexyl acetic acid
A technology of gabapentin and acetic acid, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of cyanide reactions, etc., can solve the problems of lack of a lot of research, cumbersome, cumbersome steps, etc., and achieves good atomic economy and is conducive to recovery and circulation. The effect of utilizing and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
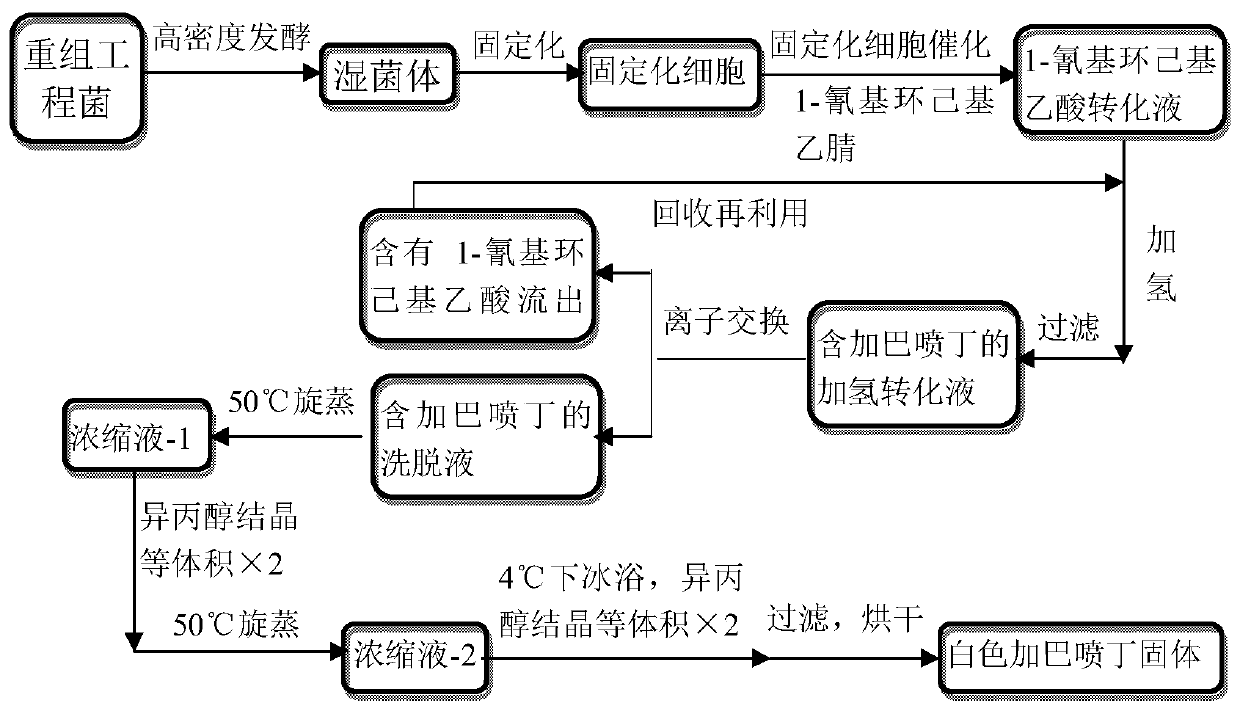
[0039] Embodiment 1: a kind of method of chemical-enzymatic synthesis of gabapentin, the steps are as follows:
[0040] (1) Preparation of seed liquid: the engineered bacteria E.coli BL21(DE3) / pET28b(+)-F168V (same as patent application CN104911225A Example 1) Inoculate on LB plate medium containing a final concentration of 5g / L kanamycin, cultivate overnight at 37°C, pick a single colony and inoculate it into 100mL of LB liquid containing a final concentration of 5g / L kanamycin culture medium at 37°C and 150 rpm overnight to obtain seed liquid.
[0041] (2) Fermentation culture: inoculate seed liquid in the 5L fermentor of the 3L fermented medium that contains final concentration 5g / L kanamycin, inoculum size is 3% (v / v), and regulating aeration ratio is 1.3vvm, in Fermentation culture was carried out at 37°C and 500rpm. During the fermentation process, ammonia water with a volume concentration of 8% and an aqueous solution of phosphoric acid with a volume concentration of 1...
experiment example 2
[0047] Experimental Example 2: Direct hydrogenation of conversion solution catalyzed by immobilized cells containing 1-cyanocyclohexylacetic acid to produce gabapentin
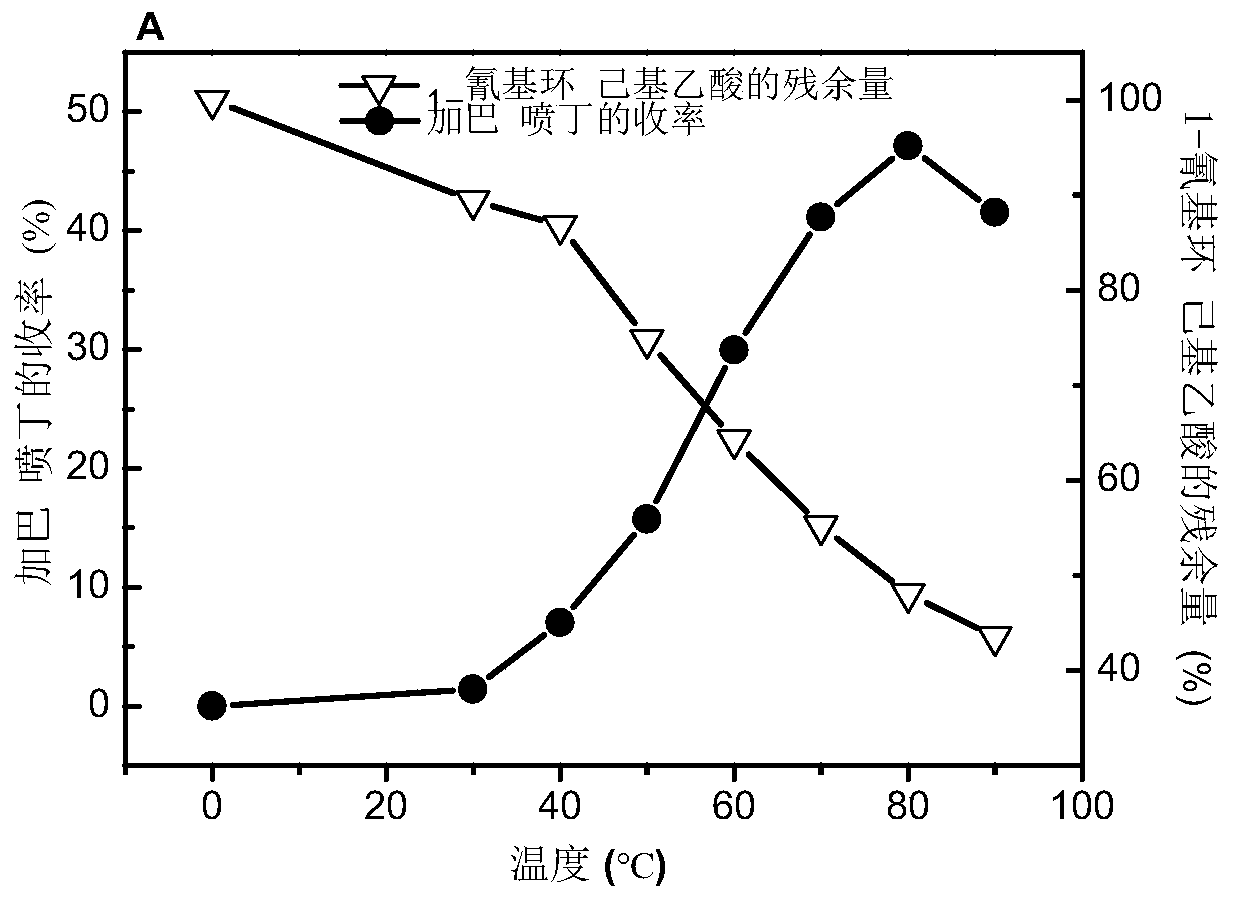
[0048] (1) Synthesis of gabapentin: get the immobilized cell catalyzed filtrate a containing 1-cyanocyclohexylacetic acid in step (4) in embodiment 1 after diluting 4 times with distilled water (1-cyanocyclohexylacetic acid concentration is 250mM ) 100mL into the hydrogenation reactor, add about 1.0g Raney Ni, add 1mL 98% triethylamine and 0.5mL 98% formic acid; replace the air with nitrogen, after 3 times, introduce 1MPa hydrogen at 30°C, 500rpm After keeping at low temperature for 12 hours, remove the hydrogen gas, raise the temperature to 40°C, keep at 500rpm for 12 hours, then remove the hydrogen gas, heat up to 50°C, then pass in the hydrogen gas, keep for 12 hours, remove the hydrogen gas, raise the temperature to 60°C, pass in the hydrogen gas, keep for 12 hours, and pass Inject hydrogen at 70°C, keep a...
experiment example 3
[0050] Experimental example 3: Direct hydrogenation of conversion solution catalyzed by immobilized cells containing 1-cyanocyclohexylacetic acid to produce gabapentin
[0051](1) Synthesis of gabapentin: get the filtrate a catalyzed by immobilized cells containing 1-cyanocyclohexylacetic acid in the step (4) of Example 1 after diluting 4 times with distilled water (the concentration of 1-cyanocyclohexylacetic acid is 250mM ) 100mL to the hydrogenation reactor, add about 1.0g Pd / C (Pd mass load 10%), 1mL 98% triethylamine and 0.5mL 98% formic acid; Inject 1MPa hydrogen at 40°C, keep at 500rpm for 12 hours, then remove the hydrogen, heat up to 50°C, then pass in hydrogen, leave for 12 hours, remove the hydrogen, raise the temperature to 60°C, pass in hydrogen, and hold for 12 hours. After degassing, the reaction liquid is filtered to obtain filtrate b and filter cake b, the filter cake b is the recovered catalyst, and the filtrate b is cooled to room temperature.
[0052] (2) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com