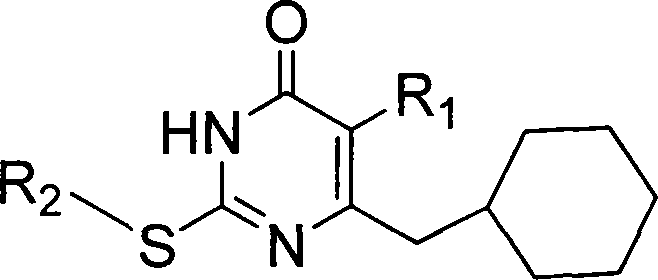
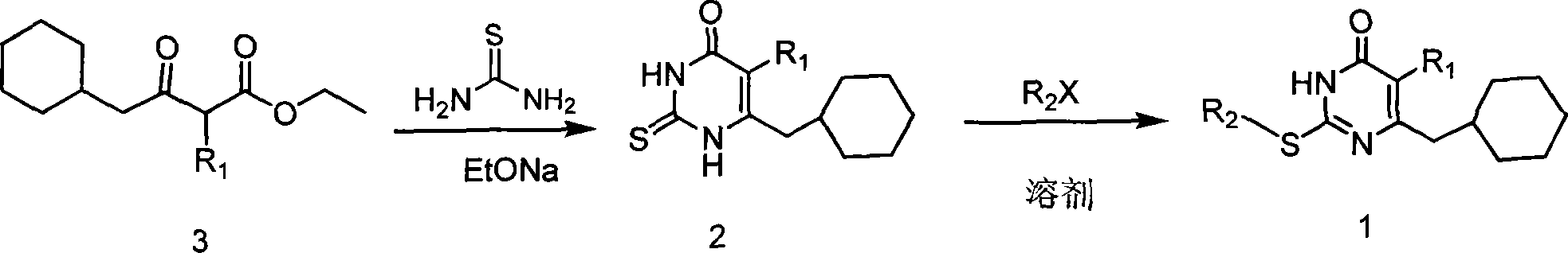
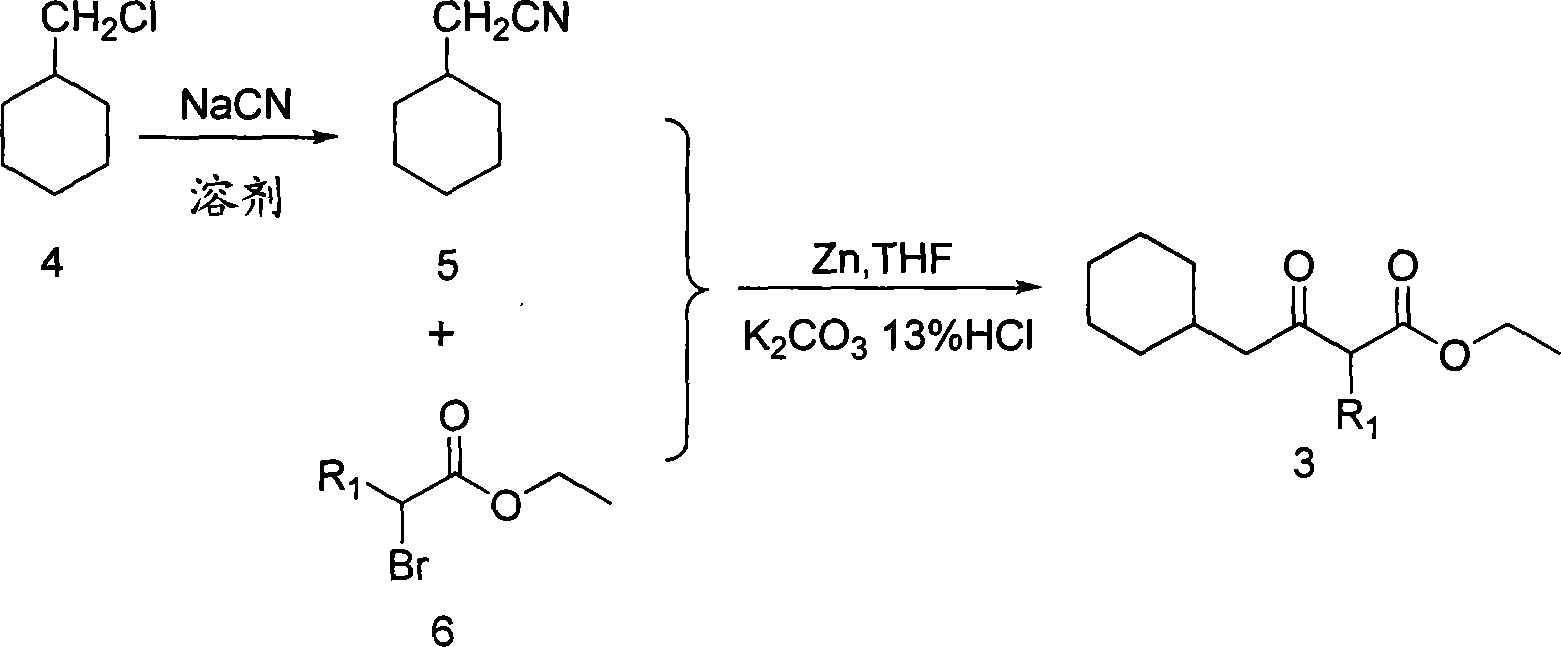
6-cyclohexyl methyl substituted s-DABO compound, method for synthesizing same and uses thereof
A cyclohexyl, compound technology, applied in the field of 6-cyclohexyl substituted S-DABO compounds, can solve the problems of drug resistance, limitation of NNRTIs antiviral potential, mutation, etc., to achieve convenient synthesis, good resistance Receptivity, strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment one β-ketoester (3)
[0035] Method 1: Preparation with chloromethylcyclohexane as raw material
[0036] The preparation of cyclohexyl acetonitrile (5): add 30ml dry ethanol, 7.35g (0.15mol) NaCN in the round bottom flask, stir until NaCN dissolves completely, add 0.1mol chloromethylcyclohexane, reflux and stir for 4 hours, Evaporate the solvent under reduced pressure, add water to dissolve, extract with ethyl acetate, combine the organic layers, and successively wash with saturated NaHCO 3 , washed with salt water, anhydrous CaCl 2 After drying, cyclohexylacetonitrile was obtained as a yellow oil.
[0037] Preparation of β-ketoester (3): activate zinc powder with 3M HCl, distilled water, absolute ethanol, and absolute ether sequentially. Activated zinc powder (45 g, 0.68 mol) was suspended in refluxing THF (400 mL) under nitrogen protection, and a few drops of 6 were added thereto to initiate the reaction. After the green color appeared ...
Embodiment 2
[0042] The synthesis of embodiment two 5-alkyl-6-cyclohexylmethylthiouracil (2)
[0043] General operation of the reaction: Add 50ml of absolute ethanol and 0.6g of metallic sodium (26.2mmol) into a round bottom flask to prepare a sodium alkoxide solution. Add thiourea (1.39g, 18.2mmol) and compound 3 (13.1mmol), stir under reflux, cool down after TLC traces the disappearance of the raw material point, evaporate ethanol under reduced pressure, dissolve the residue with 20ml of water, precipitate out after acidification with HCl, and filter with suction , washed the precipitate with water and diethyl ether, dried in vacuo, and recrystallized with an appropriate solvent to obtain 5-alkyl-6-cyclohexylmethylthiouracil (2).
Embodiment 3
[0044] Example 3 Synthesis of 5-alkyl-6-cyclohexylmethyl-2-substituted uracil (1)
[0045] General operation of the reaction: 5-alkyl-6-cyclohexyl-2-thiouracil (2) (3mmol) and K 2 CO 3 (3.3mmol) was dissolved in 10ml of anhydrous DMF, stirred at room temperature for 30min, added bromide (3.3mmol), continued stirring reaction at 70-80°C, followed by TLC, until the raw material point disappeared (12-24h), stop the reaction , filtered, and the solvent was evaporated under reduced pressure, and the residue was dissolved in 30ml of dichloromethane, washed with saturated brine, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain oily or solid crude products, which were purified by column chromatography to obtain pure compounds of various 5-alkyl-6-cyclohexylmethyl-2-substituted uracils (1).
[0046]
[0047] Operation as above, column chromatography separation (P:E=2:1) to obtain white powder 1a, yield: 54%; melting point: 190-192°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com