Nitrilase mutant and application thereof in preparation of anti-epileptic drug intermediate
A technology of nitrilase and mutants, applied in the directions of immobilization on or in inorganic carriers, hydrolase, immobilization on/in organic carriers, etc., can solve the problems of poor thermal stability and low catalytic activity of catalyst enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Semi-rational design and site-directed mutation
[0039] To contain the nitrilase gene AcN-M cloned in Acidovorax facilis (A.facilis) CCTCC NO: M 209044 (shown in the nucleotide sequence of SEQ ID NO.1, the encoded protein amino acid sequence is shown in SEQ ID NO.2 )'s pET-28b(+)-AcN-M plasmid as a template, calculate the sites that can improve thermal stability through http: / / kazlab.umn.edu / , and then perform site-directed mutation of the whole plasmid (Table 1) PCR amplification . PCR reaction system (50 μL): template 0.5-20ng, 2×Phanta max Buffer 25 μL, 0.2 mM dNTP, primers 0.2 μM, Phanta Max Super-Fidelity DNA Polymerase 1 μL, add water to make up to 50 μL. PCR conditions: (1) Pre-denaturation at 95°C for 3 minutes; (2) Denaturation at 95°C for 15 seconds; (3) Annealing at 60°C for 15 seconds; (4) Extension at 72°C for 5.5 minutes, steps (2) to (4) for a total of 30 cycles; (5) Finally, extend at 72°C for 10 minutes and store at 16°C. The PCR product ...
Embodiment 2
[0044] Example 2: Expression of nitrilase mutants
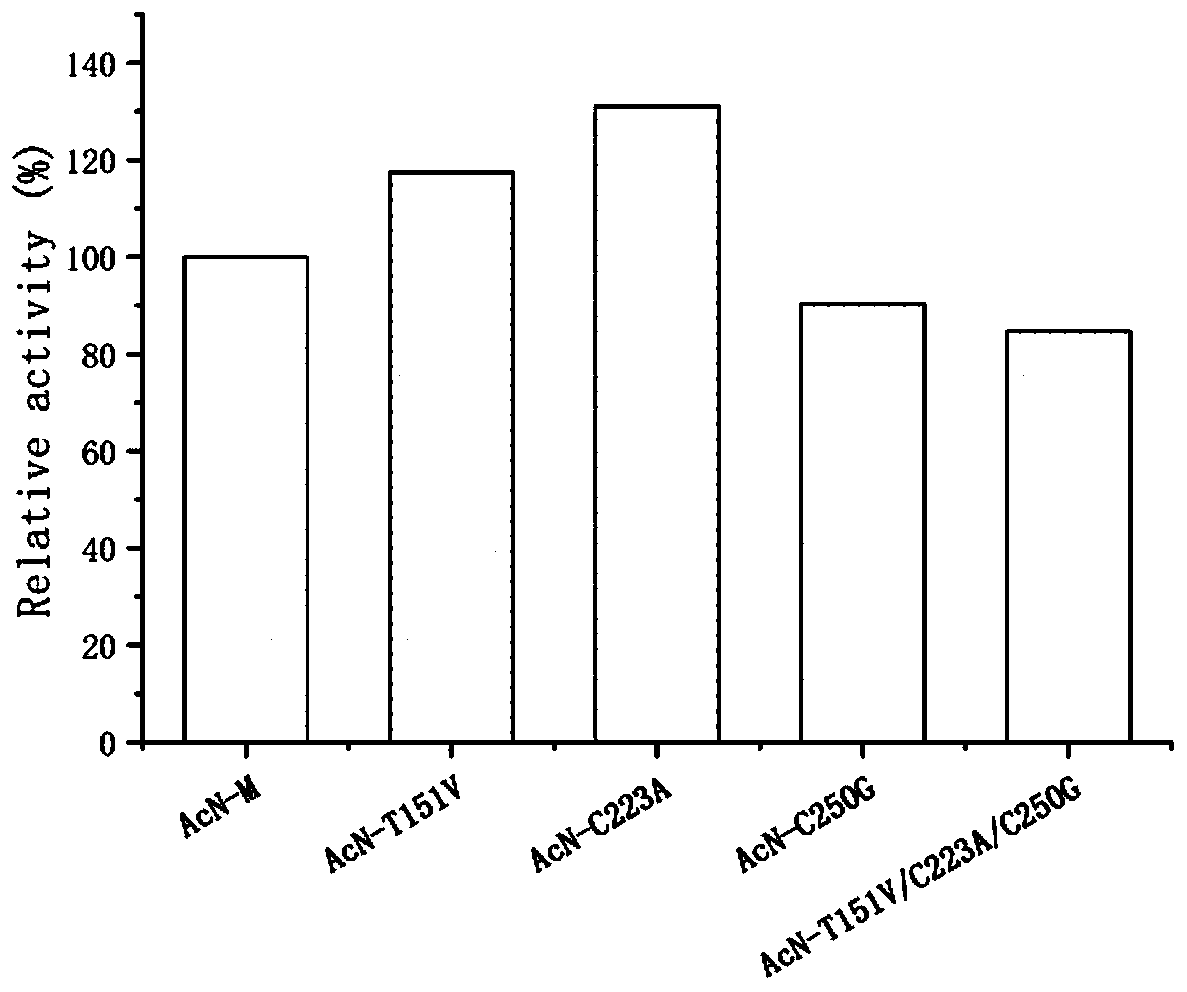
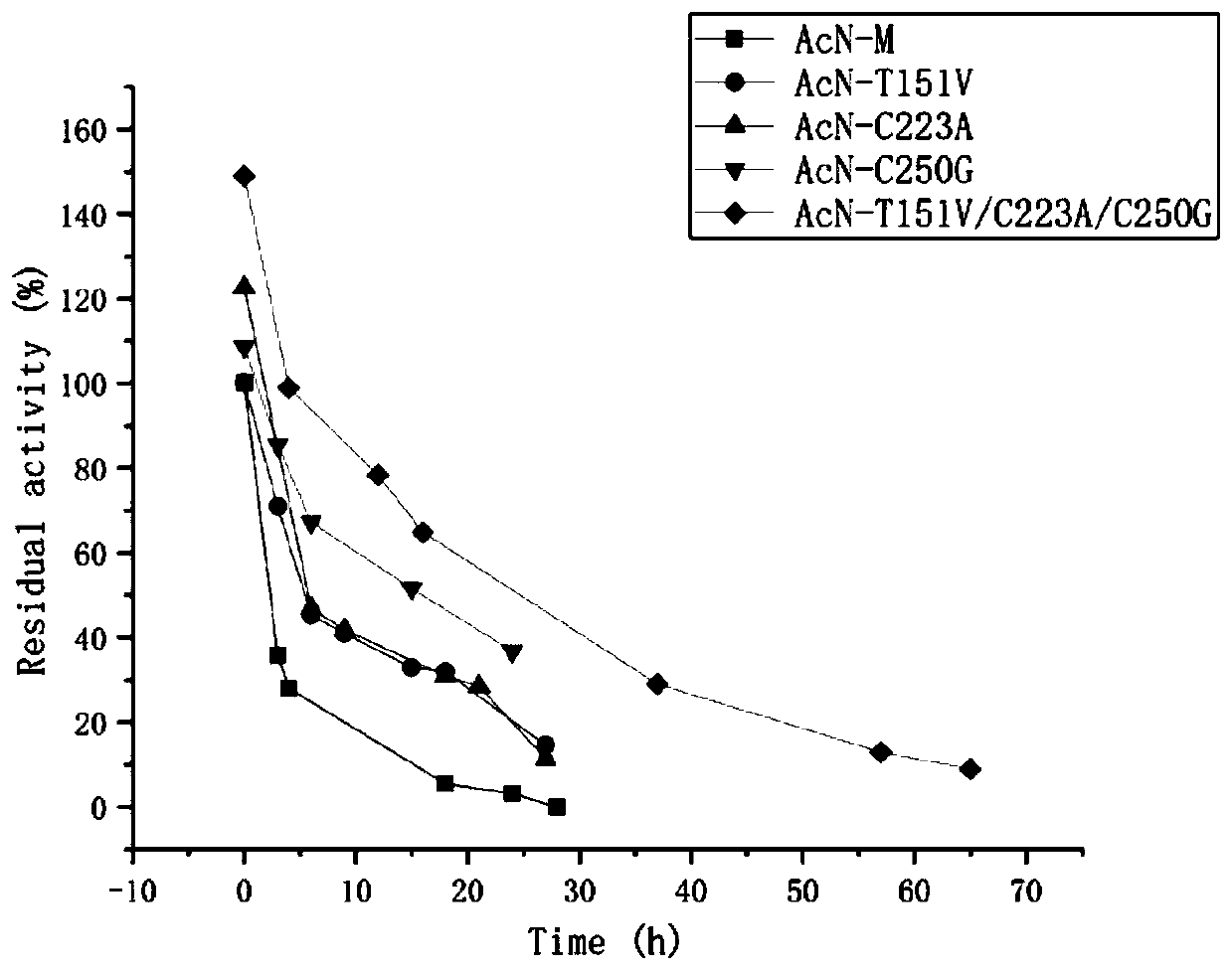
[0045] The mutant E.coli BL21(DE3) / pET28b(+)-AcN-T151V obtained in Example 1, E.coli BL21(DE3) / pET28b(+)-AcN-C223A, E.coli BL21(DE3) / pET28b(+)-AcN-C250G, and the combined mutant E.coli BL21(DE3) / pET28b(+)-AcN-T151V / C223A / C250G, and the original strain E.coli BL21(DE3) / pET28b(+)- AcN-M was inoculated into LB medium, cultured at 37°C for 10-12 hours, inoculated into LB medium containing kanamycin (final concentration 50 mg / L) at 2% inoculum volume, and expanded to culture medium at 37°C OD 600 between 0.6-0.8, add isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mM, and induce culture at 28°C for 10 hours. The bacterial cells were collected by centrifugation of the culture solution, washed twice with normal saline, and the corresponding wet bacterial cells were obtained.
Embodiment 3
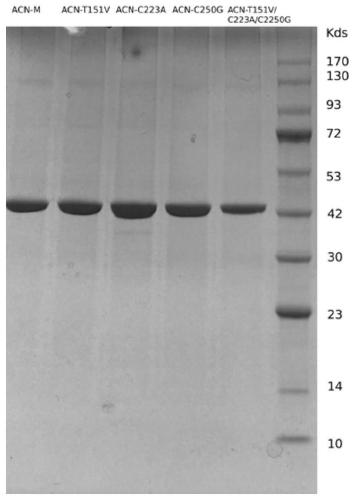
[0046] Embodiment 3: the purification of nitrilase mutant protein
[0047] (1) Add equilibrium buffer (50mM NaH 2 PO 4 , 300mM NaCl buffer, pH 8.0) after resuspending the cells, ultrasonic disruption (400W, 25min, 1s disruption 1s pause). The crushed product was centrifuged (12000×g, 10 min), and the supernatant was taken as a crude enzyme solution for separation and purification.
[0048] (2) After prepacking 20mL Ni-NTA affinity chromatography column, use equilibration buffer (50mM NaH 2 PO 4 , 300mMNaCl, pH 8.0) for equilibration at a flow rate of 2mL / min.
[0049] (3) After cleaning 8-10 column volumes, pass the obtained crude enzyme solution through the Ni-NTA column at a flow rate of 1 mL / min, and the target protein is mounted on the chromatography column. After loading, a large number of unadsorbed foreign proteins will not be bound to the resin and will be removed directly.
[0050] (4) Use elution buffer (50mM NaH 2 PO 4 , 300mM NaCl, 50mM imidazole, pH 8.0) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com