Pantoea amidase, gene, vector, engineering bacterium and application thereof
A technology for encoding bacteramidase and bacteramidase, which is applied in genetic engineering, application, plant genetic improvement, etc., can solve the problems of large amount of catalyst and long reaction time, and achieve short reaction time, small amount of catalyst, and high substrate concentration. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the acquisition of pantea amidase gene pa-ami
[0027] The amidase gene is synthesized by means of molecular biology. The specific screening method is to search for the amino acid sequence encoded by the gene encoded by NCBI according to the conserved sequence of amidase of the AS family and the NF family, and further screen for proteins that can match the catalytic triad . Finally, an amidase (GenBankaccessionNO.WP_008109374) derived from Pantoeasp.YR343 was established, the amino acid sequence of which is shown in SEQ ID NO:1. According to the preferred codon of Escherichia coli, the amidase gene was optimized, and the amidase gene pa-ami was synthesized by the conventional operation of genetic engineering, with a full length of 1445bp (its nucleotide sequence is as SEQ ID NO: 2 shown), the sequence encodes a complete open reading frame.
Embodiment 2
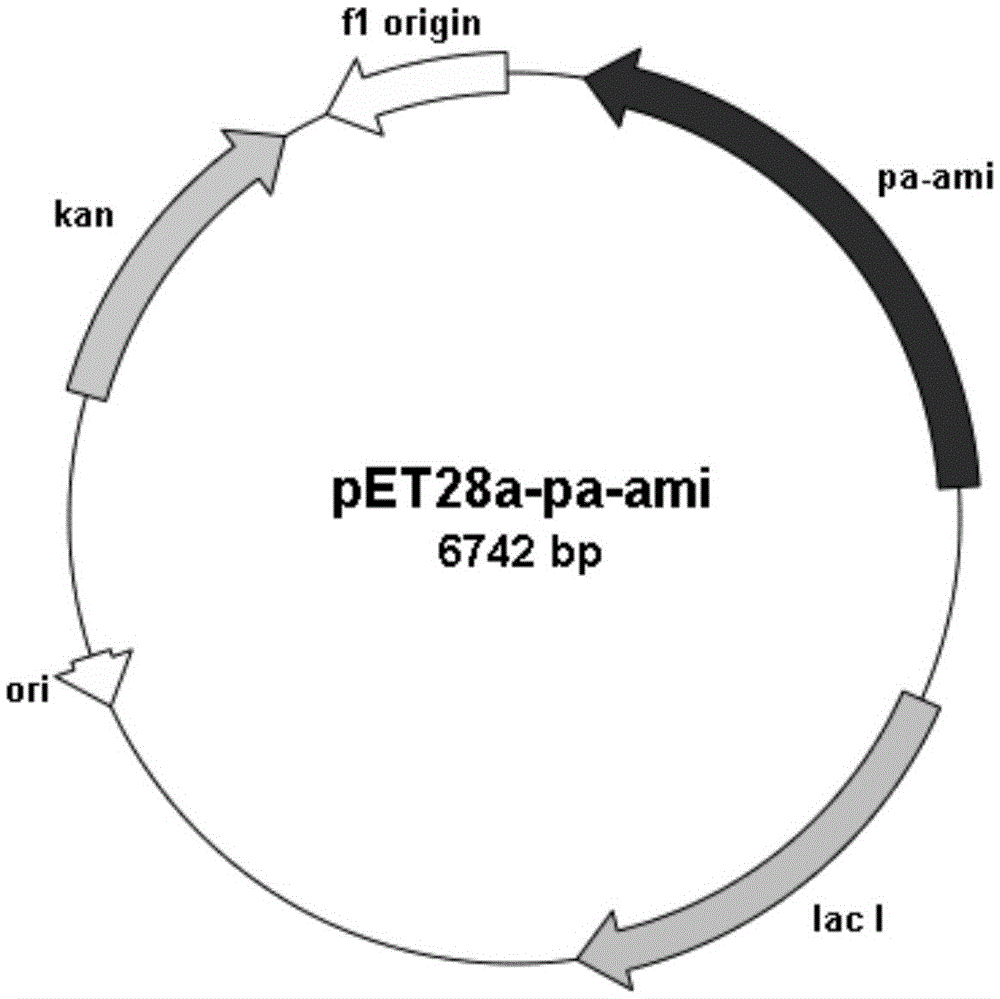
[0028] Embodiment 2: Construction of recombinant expression vector pET28a-Pa-Ami
[0029] The pa-ami fragment of the amidase gene synthesized in Example 1 was connected to the pUC57 vector by using the PMD-18T kit of TaKaRa Company to obtain the cloning recombinant plasmid pUC57-Pa-Ami. The recombinant plasmid was transformed into Escherichia coli JM109, a single colony was randomly picked and sequenced, and the gene whose nucleotide sequence was shown in SEQ ID NO:2 was obtained.
[0030] Cultivate E.coliJM109 cells containing the recombinant plasmid pUC57-Pa-Ami, use the plasmid extraction kit to extract the plasmid pUC57-Pa-Ami, perform double digestion with restriction endonuclease NcoI / HindⅢ (Fermentas), and recover after gel cutting The fragment was ligated overnight with the commercial vector pET-28a (Novagen) treated with the same restriction endonuclease using T4 ligase (Promega) to construct the recombinant expression plasmid pET28a-Pa-Ami.
Embodiment 3
[0031] Embodiment 3: Construction of genetically engineered bacteria E.coliBL21 / pET28a-Pa-Ami
[0032] The recombinant expression vector pET28a-Pa-Ami constructed in Example 2 was transformed into Escherichia coli BL21(DE3), spread on an LB plate containing a final concentration of 50 μg / mL kanamycin, and cultured overnight at 37°C. Clones were randomly selected for colony PCR identification, and positive clones were sequenced for verification. The results showed that the recombinant expression vector pET28a-Pa-Ami was successfully transformed into the expression host E.coliBL21(DE3), and the amidase gene was successfully cloned into the NcoI and HindⅢ sites of pET-28a, and the recombinant genetically engineered bacteria E.coliBL21( DE3) / pET28a-Pa-Ami.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com