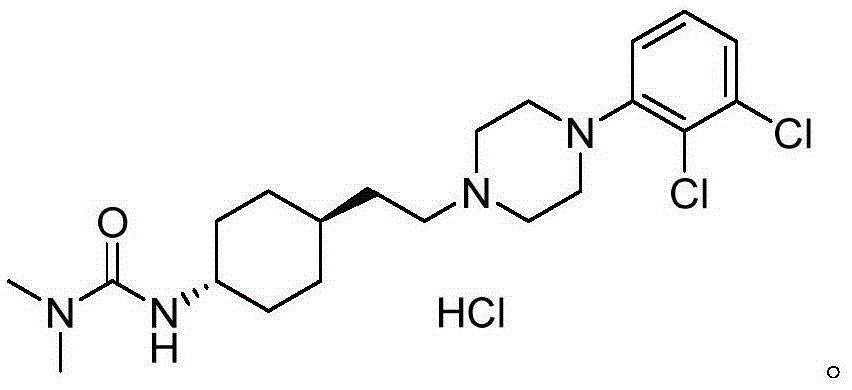
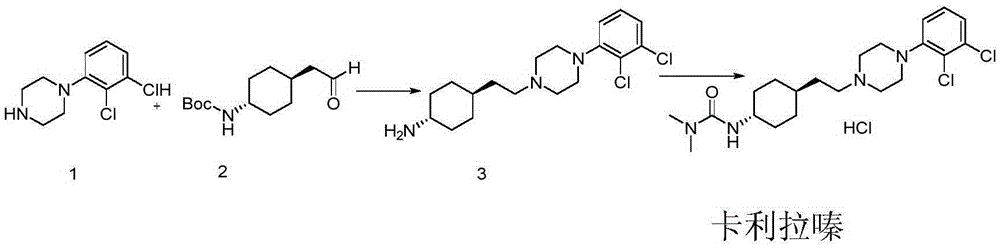
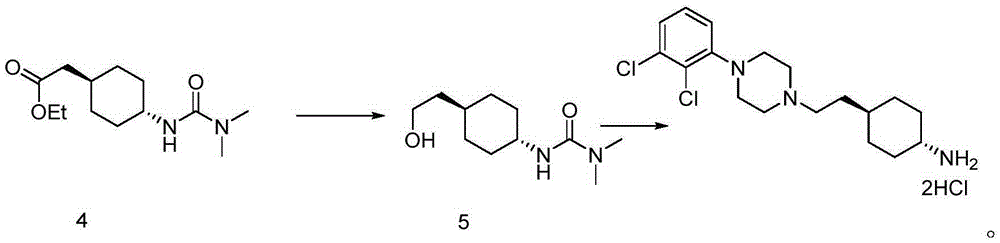
Preparation method for trans 4-amino-cyclohexyl acetate derivative
A technology of cyclohexyl acetate and aminocyclohexanone, which is used in the preparation of pharmaceutical intermediates and the preparation of trans 4-amino-cyclohexyl ethyl ester derivatives, can solve the problem that cariprazine cannot meet pharmaceutical standards , purification of trans products and other issues, to achieve the effect of great application value, easy availability of raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of trans 4-amino-cyclohexyl ethyl acetate hydrochloride
[0043]
[0044] 1L there-necked flask, add 450ml THF, nitrogen protection, ice bath, internal temperature down to 5°C, slowly add (45.0g, 0.39mol, 3.0eq) potassium tert-butoxide, stir to dissolve, dropwise add (45.0g, 0.2mol ,1.5eq) triethyl phosphoroacetate, the internal temperature is less than 10°C, after the addition is completed, rise to room temperature and stir for 0.5h, then add (14.7g, 0.13mol, 1.0eq) raw material 4-aminocyclohexanone II in batches, and react 2 hours until raw material disappears. Add 200ml of water, separate the layers, wash the organic phase with 100ml of saturated sodium chloride, dry over anhydrous sodium sulfate, and spin dry to obtain 24.2g of crude oily product of ethyl 4-amino-cyclohexyl ethylene acid, which is directly put into the next reaction.
[0045]
[0046] In a 1L single-mouth bottle, add the olefin product from the previous step (24.2g, calc...
Embodiment 2
[0048] Example 2 Preparation of trans-4-amino-cyclohexylacetic acid methyl ester hydrochloride
[0049]
[0050] 1L three-necked flask, add 50ml methyl tert-butyl ether, nitrogen protection, ice bath, the internal temperature dropped to 5 ℃, slowly add (1.14g, 14.3mmol, 1.1eq) lithium tert-butoxide, stir to dissolve, drop ( 2.6g, 14.3mol, 1.1eq) trimethyl phosphoacetate, the internal temperature is less than 10°C, after the addition is complete, raise to room temperature and stir for 0.5h, then add (1.47g, 13mmol, 1.0eq) raw material 4-aminocyclohexyl in batches Ketone II, react for 2.5 hours until the raw material disappears. Add 50ml of water, separate the layers, wash the organic phase with 50ml of saturated sodium chloride, dry over anhydrous sodium sulfate, and spin dry to obtain 2.2g of crude oily product of 4-amino-cyclohexylethylene acid methyl ester, which is directly put into the next reaction.
[0051]
[0052] 1L single-necked bottle, add the olefin product ...
Embodiment 3
[0055]
[0056] Add 45ml tetrahydrofuran to a 1L three-necked flask, protect it with nitrogen, and put it in an ice bath. The inner temperature drops to -10°C, slowly add (8.9ml, 14.3mmol, 1.1eq) n-butyllithium hexane solution (1.6M / L), and stir to dissolve After that, (8.74g, 39mol, 3eq) triethyl phosphoroacetate was added dropwise, the internal temperature was less than 10°C, after the addition was completed, it was raised to room temperature and stirred for 0.5h, and (1.47g, 13mmol, 1.0eq) was added in batches (1.47g, 13mmol, 1.0eq) raw material 4- Aminocyclohexanone, reacted for 2 hours until the disappearance of raw materials. Add 50ml of water, separate the layers, wash the organic phase with 50ml of saturated sodium chloride, dry over anhydrous sodium sulfate, and spin dry to obtain 2.41g of crude oily product of ethyl 4-amino-cyclohexyl ethylene acid, which is directly put into the next reaction.
[0057]
[0058] In a 1L single-necked bottle, add the crude olefi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com