New method for synthesizing Gabapentin hydrochloride
A new synthesis technology of gabapentin hydrochloride, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high equipment requirements, environmental pollution, poor safety, etc., and achieve industrialized production with short routes. , less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
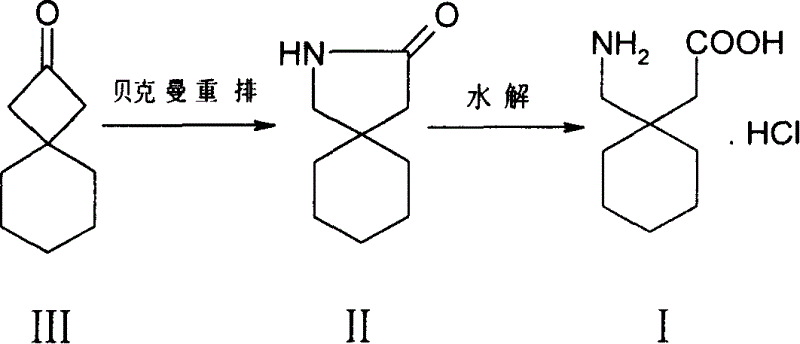
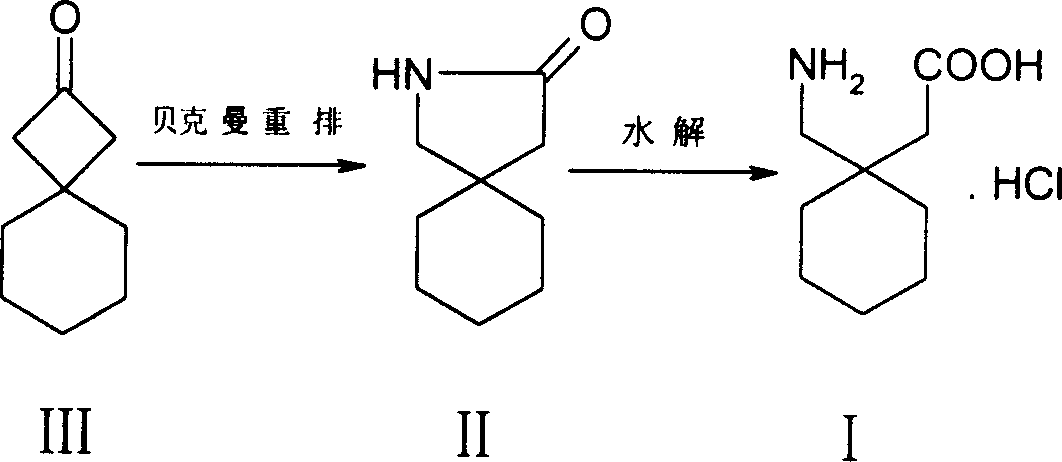
[0014] Embodiment 1: the preparation of spiro[3,5]-2-nonanone III
[0015] To methylenecyclohexane IV (0.96g, 10mmol), zinc powder (0.768g, 12mmol), 40mL ether solution, dropwise add trichloroacetyl chloride (2.2g, 12mmol) 10mL ether solution, keep the temperature at 20°C , continue ultrasonication for 3-4 hours, the temperature is gradually raised from 20°C to 40°C, and excess zinc powder is filtered off, the filtrate is washed with saturated ammonium chloride solution, sodium bicarbonate solution, and salt water, and diethyl ether is evaporated with a rotary evaporator. 1.94 g of the compound 1,1-dichlorospiro[3,5]-2-nonanone was obtained.
[0016] The compound 1,1-dichlorospiro[3,5]-2-nonanone (1.94g, 9.35mmol) and zinc powder (1.92g, 30.mmol) were stirred in 20mL of glacial acetic acid at 20-30°C for 10 hours, Thin-layer chromatography TLC showed that the substrate disappeared, filtered with suction, added 50 mL of water to the filtrate, extracted three times with n-hexan...
Embodiment 2
[0022] The preparation of spiro[3,5]-2-nonanone III is the same as in Example 1
[0023] Preparation of 2-aza-spiro[4,5]-3-decanone II
[0024] At room temperature, hydroxylamine-O-sulfonic acid (0.992g, 7.5mmol) was slowly added to a solution of compound III (765mg, 5mmol) in 10mL of formic acid, stirred until the solution was transparent, heated to reflux for 4.5 hours, thin layer chromatography TLC It showed that compound III disappeared, cooled to room temperature, poured into 50mL saturated sodium bicarbonate solution, adjusted to weak alkalinity, extracted with chloroform (15mL×3), washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, and used a rotary evaporator The solvent was distilled off to obtain a yellow oil, which was subjected to column chromatography (eluent: n-hexane: ethyl acetate = 3:1) to obtain 0.4 g of compound II.
[0025] The preparation of 1-(aminomethyl)-cyclohexylacetic acid hydrochloride I
[0026] Compound 2-aza-s...
Embodiment 3
[0028] The preparation of spiro[3,5]-2-nonanone III is the same as in Example 1
[0029] Preparation of 2-aza-spiro[4,5]-3-decanone II
[0030] At room temperature, hydroxylamine-O-sulfonic acid (1.32g, 10mmol) was slowly added to a solution of compound III (765mg, 5mmol) in 10mL of formic acid, stirred until the solution was transparent, heated to reflux for 4 hours, thin layer chromatography TLC showed Compound III disappeared, cooled to room temperature, poured into 50ml of saturated sodium bicarbonate solution, adjusted to weak alkalinity, extracted with chloroform (15ml×3), washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, evaporated with a rotary evaporator The solvent was removed to obtain a yellow oil, which was subjected to column chromatography (eluent: n-hexane: ethyl acetate = 3:1) to obtain 0.42 g of compound II.
[0031] The preparation of 1-(aminomethyl)-cyclohexylacetic acid hydrochloride I
[0032] Compound 2-aza-spiro[4,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com