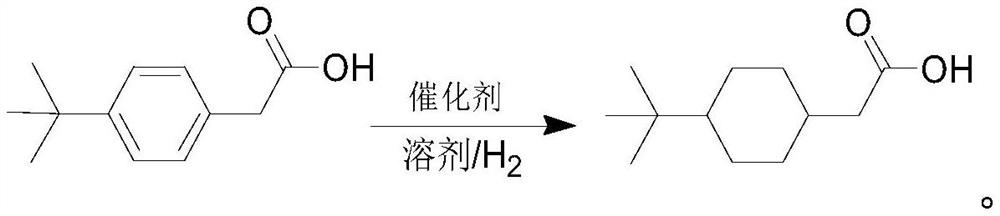
Synthesis method of 4-tert-butyl cyclohexyl acetic acid
A synthesis method and technology of tert-butylbenzene are applied in the field of synthesis of pharmaceutical intermediates, and can solve the problems such as inability of reaction, large phosphorus-containing waste water, high environmental protection pressure, etc., and achieve easy satisfaction of equipment conditions, low production cost, and reaction steps. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 50.00g (0.26mol) of 4-tert-butylphenylacetic acid in 250ml of methanol, add the solution to the hydrogenation kettle, add 5.00g of palladium-carbon catalyst to the solution, close the cover, ensure that it is firmly fixed, and then replace it with 0.3MPa nitrogen in turn 3 times, 0.3MPa hydrogen replacement 2 times, after the replacement, fill the kettle with 6MPa hydrogen and start stirring. Observe the change of the pressure gauge during the process. When the pressure in the kettle is lower than 4MPa, hydrogen is added to 6MPa, and the pressure in the straight kettle is basically stable. After the pressure in the kettle is stabilized, stop stirring, let it stand for 10 minutes, open the exhaust valve to empty the pressure in the kettle, replace it with 0.3MPa nitrogen twice, then empty the pressure in the kettle, open the lid of the kettle to mix and filter the kettle, and the obtained filtrate Concentrate by vacuum precipitation to about 100ml.
[0040] Slo...
Embodiment 2
[0042] Dissolve 50.00g (0.26mol) of 4-tert-butylphenylacetic acid in 250ml of methanol, add the solution to the hydrogenation kettle, add 15.00g of Raney nickel to the solution, close the cover, ensure that it is firmly fixed, and then replace it with 0.3MPa nitrogen in turn 3 times, 0.3MPa hydrogen replacement 2 times, after the replacement, fill the kettle with 6MPa hydrogen and start stirring. Observe the change of the pressure gauge during the process. When the pressure in the kettle is lower than 4MPa, hydrogen is added to 6MPa, and the pressure in the straight kettle is basically stable. After the pressure in the kettle is stabilized, stop stirring, let it stand for 10 minutes, open the exhaust valve to empty the pressure in the kettle, replace it with 0.3MPa nitrogen twice, then empty the pressure in the kettle, open the lid of the kettle to mix and filter the kettle, and the obtained filtrate Concentrate by vacuum precipitation to about 100ml.
[0043] Slowly pour the...
Embodiment 3
[0046] Dissolve 50.00g (0.26mol) of 4-tert-butylphenylacetic acid in 250ml of ethanol, add the solution to the hydrogenation kettle, add 15.00g of Raney nickel to the solution, close the cover, ensure that it is firmly fixed, and then replace it with 0.3MPa nitrogen in turn 3 times, 0.3MPa hydrogen replacement 2 times, after the replacement, fill the kettle with 6MPa hydrogen and start stirring. Observe the change of the pressure gauge during the process. When the pressure in the kettle is lower than 4MPa, hydrogen is added to 6MPa, and the pressure in the straight kettle is basically stable. After the pressure in the kettle is stabilized, stop stirring, let it stand for 10 minutes, open the exhaust valve to empty the pressure in the kettle, replace it with 0.3MPa nitrogen twice, then empty the pressure in the kettle, open the lid of the kettle to mix and filter the kettle, and the obtained filtrate Concentrate by vacuum precipitation to about 100ml.
[0047] Slowly pour the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com