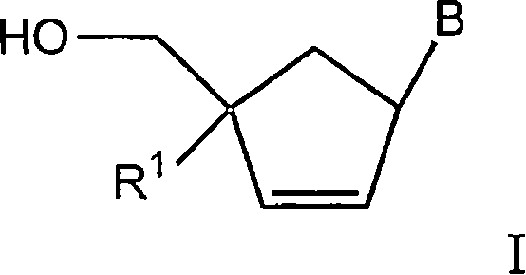
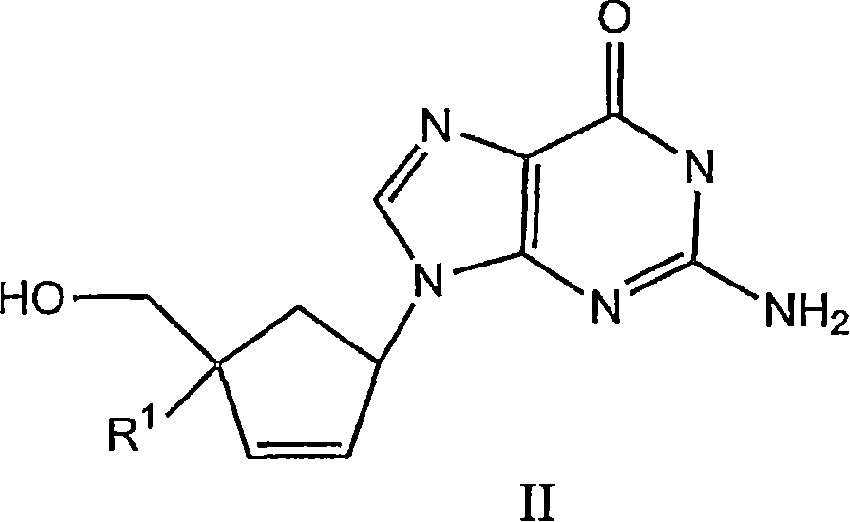
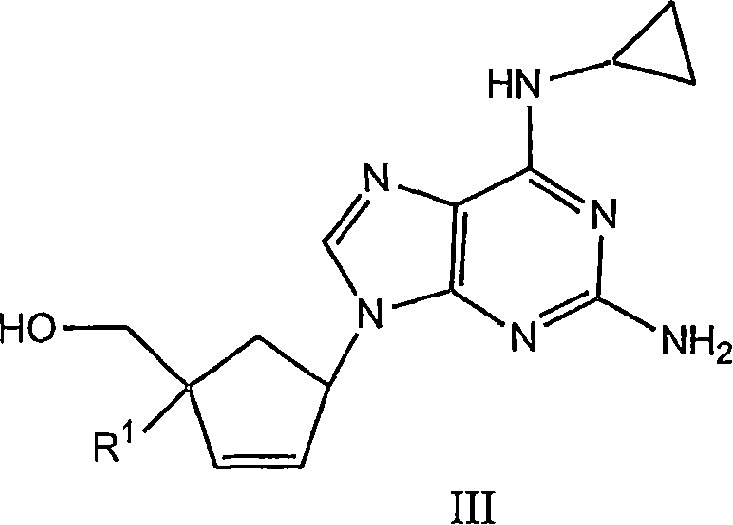
4'-substituted carbovir-and abacavir-derivatives as well as related compounds with HIV and HCV antiviral activity
A compound and solvate technology, applied in the field of 4'-substituted nucleoside derivatives, can solve problems such as occurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Embodiment 1. Preparation of typical compounds of the present invention
[0187]
[0188] Intermediate epoxides 1.11 can be prepared as follows. Selective iodination of carbovir at the 5′-position using triphenylphosphine, iodine and pyridine or imidazole in dioxane (Maag, H. et al., J. Med. Chem., 1992, 35, 1440) 1.8 (Crimmins, M.T. et al., J.Org.Chem., 1996, 61, 4192) yielded compound 1.9. The dehydrohalogenation of 5'-halogenated nucleosides has been well described (Ueda, T.In Chemistry of Nucleosides and Nucleotides; Townsend, L.B., Ed.; Plenum Press; New York, 1988; 83). The product 1.10 was prepared using sodium methoxide. Non-stereoselective epoxidation of exocyclic olefins using m-chloroperbenzoic acid or Corey's reagent (dimethylmethylene sulfoxide) (Gololobov, Y.G. et al., Tetrahedron, 1997, 43, 12, 2609), A mixture of two diastereomeric epoxides 1.11 was obtained.
[0189] Compound 1, a typical compound of the present invention, can be prepared from ep...
Embodiment 2
[0194] Embodiment 2. Preparation of typical compounds of the present invention
[0195]
[0196] Abacavir 2.8 can be prepared from compound 1.7 as described by Crimmins, M.T. et al., J. Org Chem., 1996, 61, 4192. Selective iodination at the 5'-position using triphenylphosphine, iodine, and pyridine or imidazole in dioxane (Maag, H. et al., J. Med. Chem., 1992, 35, 1440) yields Compound 2.9. The dehydrohalogenation of 5'-halogenated nucleosides has been well described (Ueda, T. In Chemistry of Nucleosides and Nucleotides; Townsend, L.B., Ed.; Plenum Press; New York, 1988; 83). Compound 2.10 was prepared using sodium methoxide. Epoxidation of the exocyclic olefin of 2.10 produced a mixture of two diastereomers without stereoselection. Such reactions can be accomplished using m-chloroperbenzoic acid or Corey's reagent (dimethylmethylene sulfoxide) (Gololobov, Y.G. et al., Tetrahedron, 1997, 43, 12, 2609). As reported by Krause, N. et al., Chem. Ber., 1988, 121, 7, 1315, onl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com