Method for preparing piperidine and piperidine derivative
A technology for derivatives, piperidine, which is applied in the field of hydrogenation to prepare piperidine and piperidine derivatives, can solve the problem of high price of raw material pyridine, improve selectivity and production capacity, increase processing capacity, and reduce preheating and cooling. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
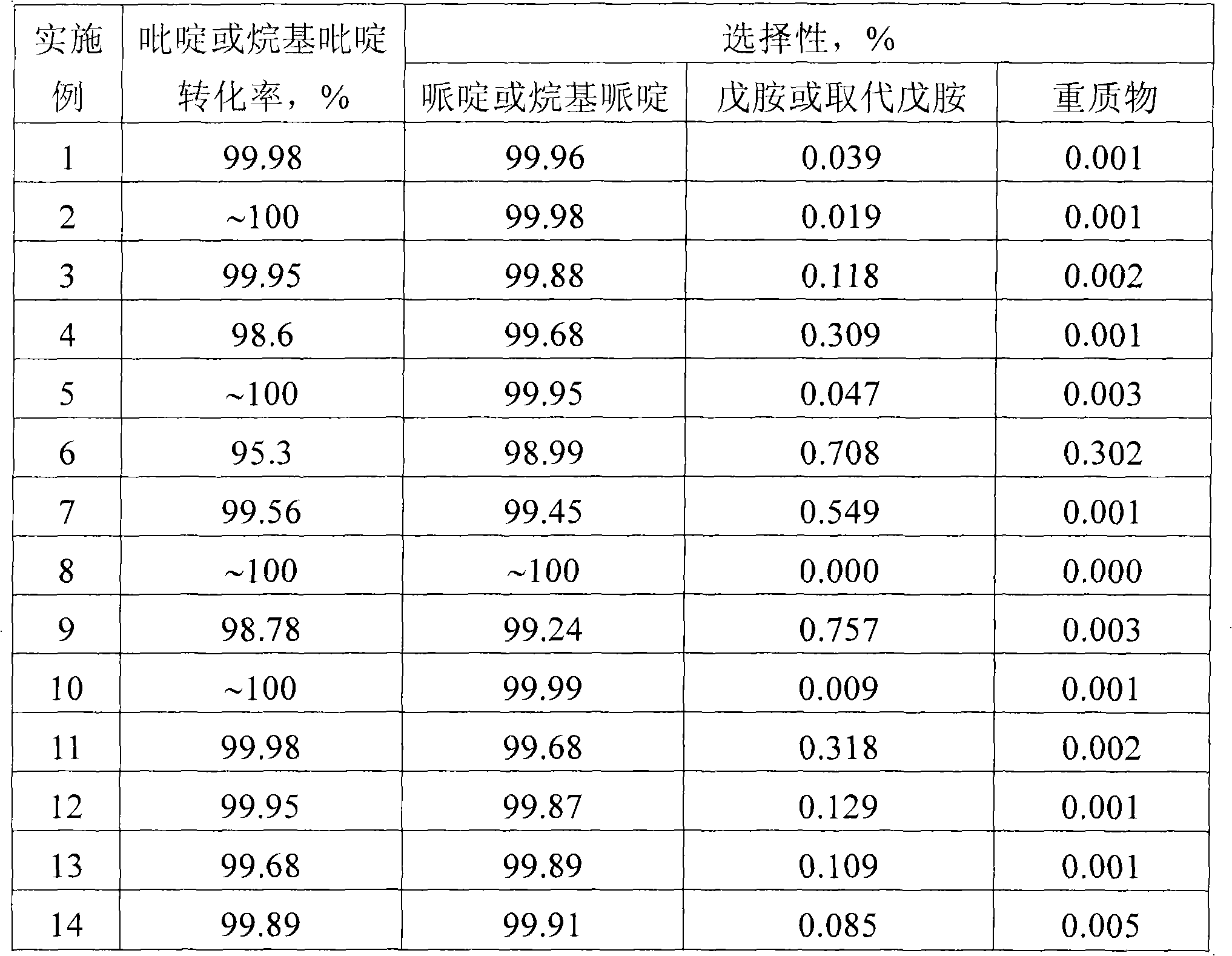
[0018] The catalyst used is Pd-Ru / Al 2 o 3 . Catalyst weight composition is: Pd=0.5% and Ru=0.003%, the rest is carrier Al 2 o 3 . The carrier adopts commercial Al 2 o 3 , and the catalyst active component Pd and additive Ru were loaded on the carrier Al by the conventional impregnation method 2 o 3 (Al 2 o 3 The particle size is 20-40 mesh). 5.0 grams (about 8ml) of the catalyst was activated by hydrogen before the reaction, and the activation conditions were: GHSV=2500h -1 , Atmospheric pressure, 300°C, reduction time 5 hours. Choose a fixed bed reactor. The reaction temperature is 150°C, the hydrogen pressure is 8.0MPa, pyridine / H 2 The molar ratio is 1:150, and the liquid space velocity of pyridine is 0.5h -1 , H 2 The reaction time is 50 hours (h), and samples are taken for analysis. Samples were analyzed by gas chromatography, HP-15 capillary column, and FID detector.
Embodiment 2
[0020] The liquid space velocity of ethanolamine is 0.35h -1 , other conditions are identical with embodiment 1.
Embodiment 3
[0022] The liquid space velocity of ethanolamine is 0.65h -1 , other conditions are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com