Method for preparing Niraparib of PARP (poly-ADP-ribose polymerase) inhibitor
A benzyl reaction technology, applied in the field of chemical medicine of the present invention, can solve the problems of long synthetic route and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
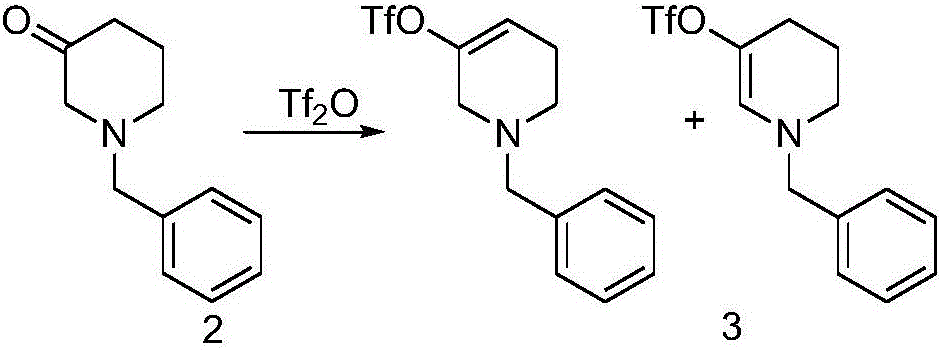
[0043] Example 1 Under the protection of nitrogen, compound 3 (36.0g, 0.19mol) was dissolved in 190mL of dichloromethane, then diisopropylethylamine (27.0g, 0.21mol) was added, and the temperature was lowered to -60°C, dropwise Add trifluoromethanesulfonic anhydride (59.2g, 0.21mol), and react at this temperature. After the reaction, add water, warm up to room temperature, extract with ethyl acetate, wash with saturated sodium bicarbonate, dry the organic phase, and evaporate under reduced pressure. The solvent was removed to obtain 44.5 g of a mixture of compound 3 as a light yellow solid.
[0044]
example 2
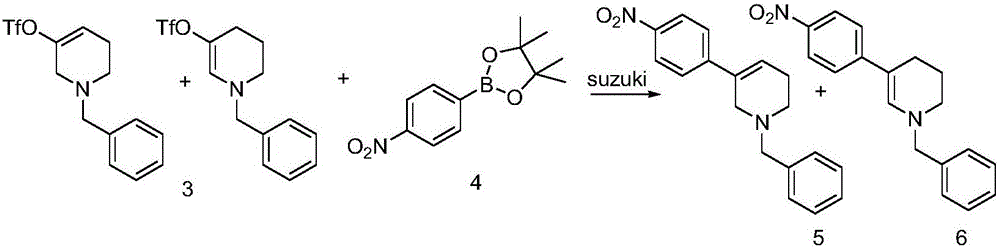
[0045] Example 2 Under the protection of nitrogen, after adding 130mL tetrahydrofuran to the mixture of compound 3 (39.0g, 0.12mol) to dissolve, add p-nitrophenyl borate (31.4g, 0.13mol), tetrakistriphenylphosphopalladium (6.9 g, 6.0mmol), potassium carbonate (33.1g, 0.13mol), and heated to reflux temperature, and reacted at this temperature for 8 hours, after the reaction, the system was lowered to room temperature, and the reaction solution was filtered with diatomaceous earth, and the filtrate Part of the solvent was distilled off under reduced pressure, crystals were precipitated at low temperature, and the mixture of compounds 5 and 6 was obtained by filtration as 37.5 g of a light brown solid.
[0046]
example 3
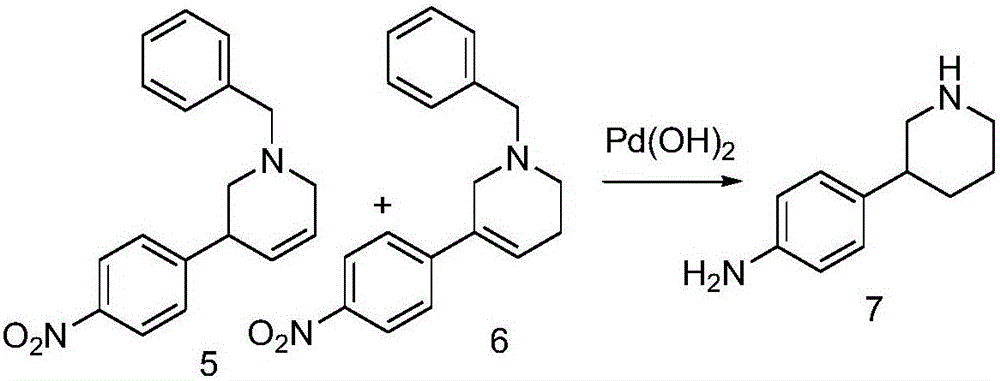
[0047] Example 3 In an autoclave, 110 mL of methanol was added to the mixture of compounds 5 and 6 (19.0 g, 0.065 mol), 30 mL of acetic acid was added, 1.5 g of palladium hydroxide was added, and then hydrogen gas was introduced, and the reaction was carried out at 12 atmospheres for 24 hours , after the reaction is complete, filter out the solid, and evaporate most of the methanol in the filtrate under reduced pressure, add 1mL sodium hydroxide solution 130mL, stir for 20 minutes, extract twice with ethyl acetate (2*100mL), and use Wash once with saturated citric acid (100 mL), dry the organic phase over anhydrous sodium sulfate, and distill the organic phase under pressure to obtain 9.3 g of a light yellow solid.
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com