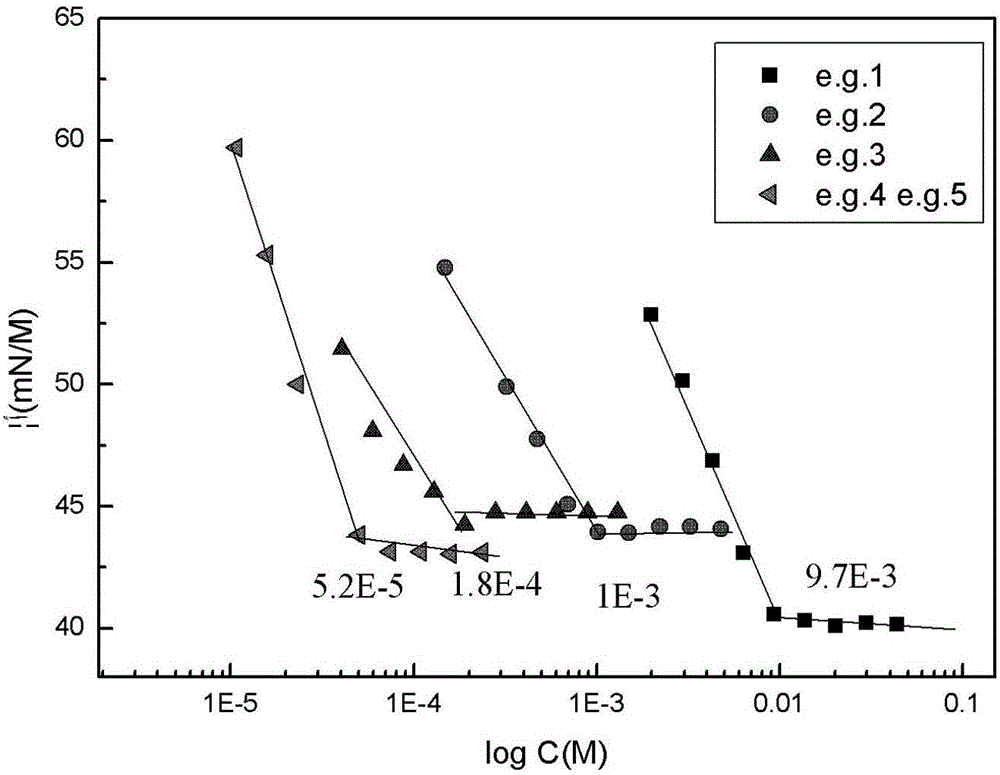
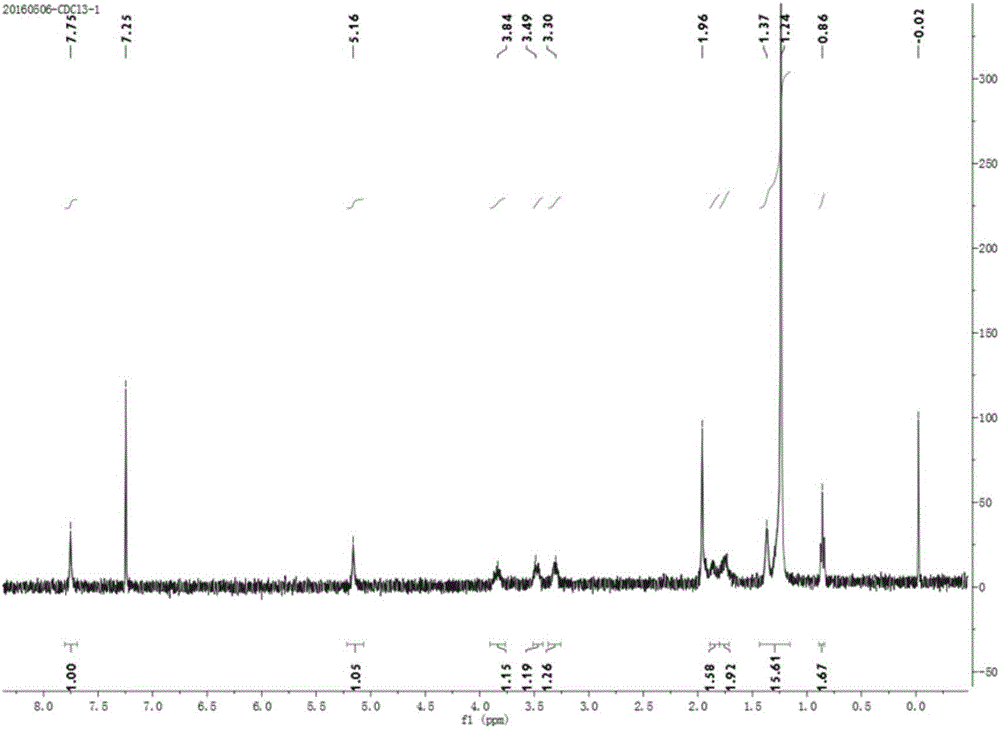
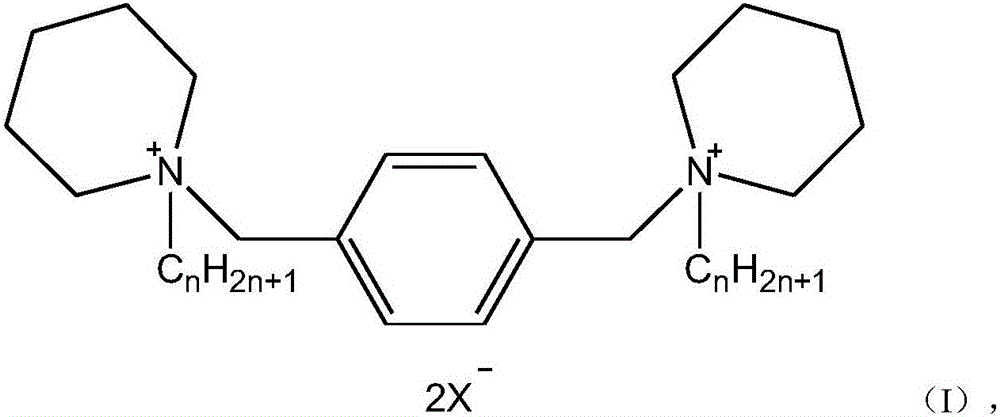
Cyclic quaternary ammonium salt gemini surfactant and preparation method thereof
A quaternary ammonium salt gemini and surfactant technology, applied in chemical instruments and methods, dissolution, organic chemistry, etc., can solve the problems of high cost, environmental pollution, health of operators, threats, etc., and achieve low pollution, solubility and Low biological toxicity and good surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of heterocyclic quaternary ammonium salt gemini surfactant, wherein n=8, X=Cl, the preparation method comprises the following steps:
[0036] 1) Intermediate product alkyl substituted piperidine C 5 h 10 N-C 8 h 17 Synthetic steps: mix 1-bromooctane and piperidine at a molar ratio of 1:3, and react at 95°C for 12 hours with absolute ethanol as a solvent; The mixture was neutralized with sodium hydroxide solution; the mixture was extracted three times with ethyl acetate, and the organic phases were combined and dried overnight with anhydrous magnesium sulfate; after filtering, the ethyl acetate in the filtrate was removed with a rotary evaporator to obtain a light yellow oily liquid, and finally Obtain high-purity alkyl-substituted piperidine (C 5 h 10 N-C 8 h 17 ), the yield is 95.1%;
[0037] 2) the synthetic steps of heterocyclic quaternary ammonium salt gemini surfactant: p-dichlorobenzyl and C 5 h 10 N-C 8 h 17 Mix according to the molar ratio of ...
Embodiment 2
[0040] A kind of heterocyclic quaternary ammonium salt gemini surfactant, wherein n=10, X=Cl, the preparation method comprises the following steps:
[0041] 1) Intermediate product alkyl substituted piperidine C 5 h 10 N-C 10 h 21 Synthetic steps: mix 1-bromodecane and piperidine at a molar ratio of 1:3.5, and react with absolute ethanol at 95°C for 24 hours; The mixture was neutralized with sodium hydroxide solution; the resulting mixture was extracted three times with ethyl acetate, and the organic phases were combined and dried overnight with anhydrous magnesium sulfate; after filtering, the ethyl acetate in the filtrate was removed with a rotary evaporator to obtain a light yellow oily liquid, Finally obtain high-purity alkyl substituted piperidine (C) with oil pump decompression distillation 5 h 10 N-C 10 h 21 ), the yield is 93.3%;
[0042] 2) the synthetic steps of heterocyclic quaternary ammonium salt gemini surfactant: p-dichlorobenzyl and C 5 h 10 N-C 10 h...
Embodiment 3
[0045] A kind of heterocyclic quaternary ammonium salt gemini surfactant, wherein n=14, X=Cl, the preparation method comprises the following steps:
[0046] 1) Intermediate product alkyl substituted piperidine C 5 h 10 N-C 14 h 29 Synthesis steps of 1-bromotetradecane and piperidine are mixed at a molar ratio of 1:3, and reacted at 95°C for 48 hours with absolute ethanol as a solvent; after the reaction is completed, the absolute ethanol is removed with a rotary evaporator, The residue was neutralized with sodium hydroxide solution; the resulting mixture was extracted three times with ethyl acetate, and the organic phases were combined and dried overnight with anhydrous magnesium sulfate; after filtration, the ethyl acetate in the filtrate was removed with a rotary evaporator to obtain a light yellow oil Liquid, finally obtain high-purity alkyl substituted piperidine (C 5 h 10 N-C 14 h 29 ), the yield is 95.3%;
[0047] 2) the synthetic steps of heterocyclic quaternary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com