Use of chiral oxazoline
A chiral oxazoline, oxazoline technology, applied in asymmetric synthesis, hydrogen cyanide addition preparation, organic compound/hydride/coordination complex catalyst, etc., can solve problems such as low efficiency and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
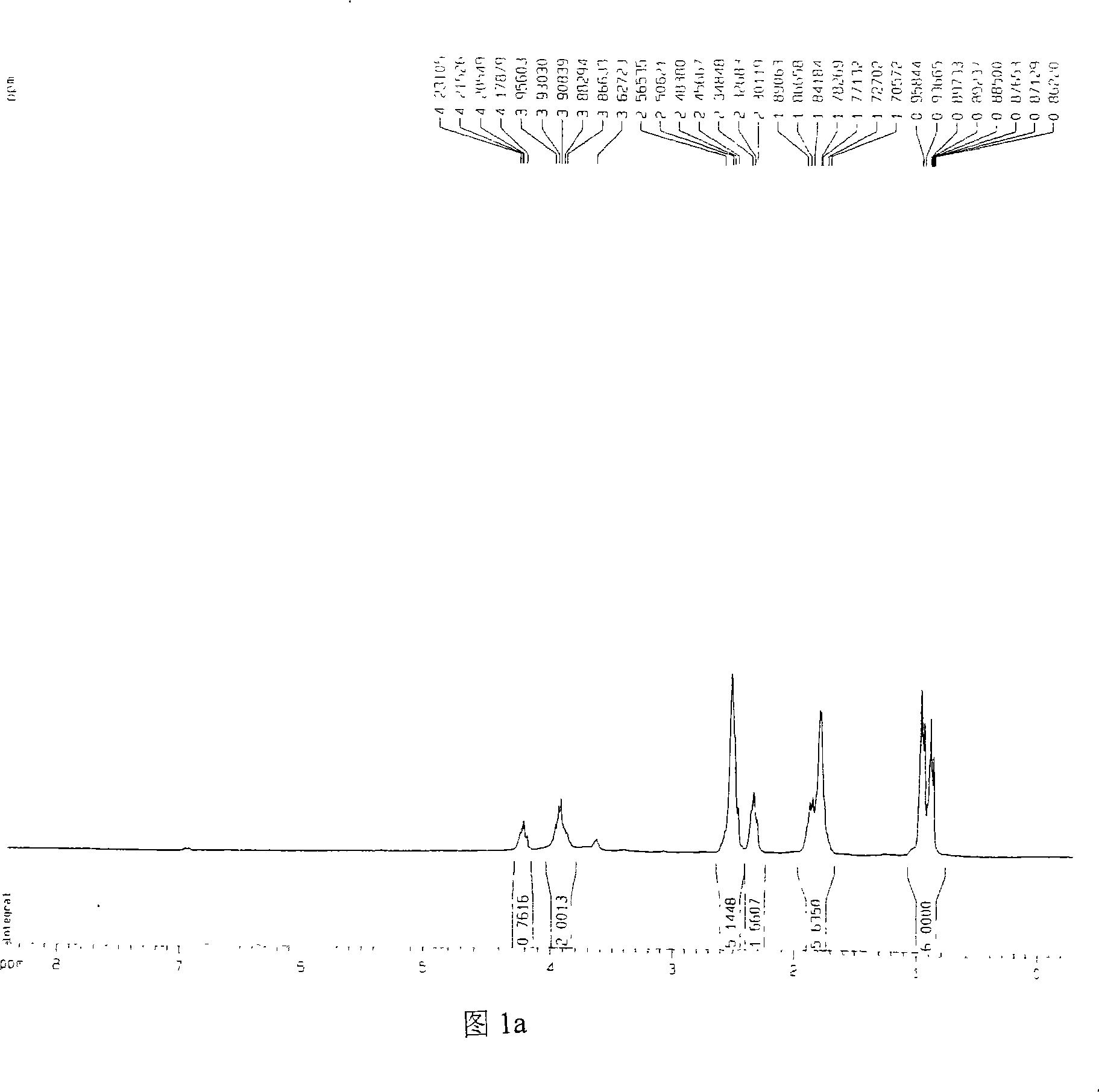
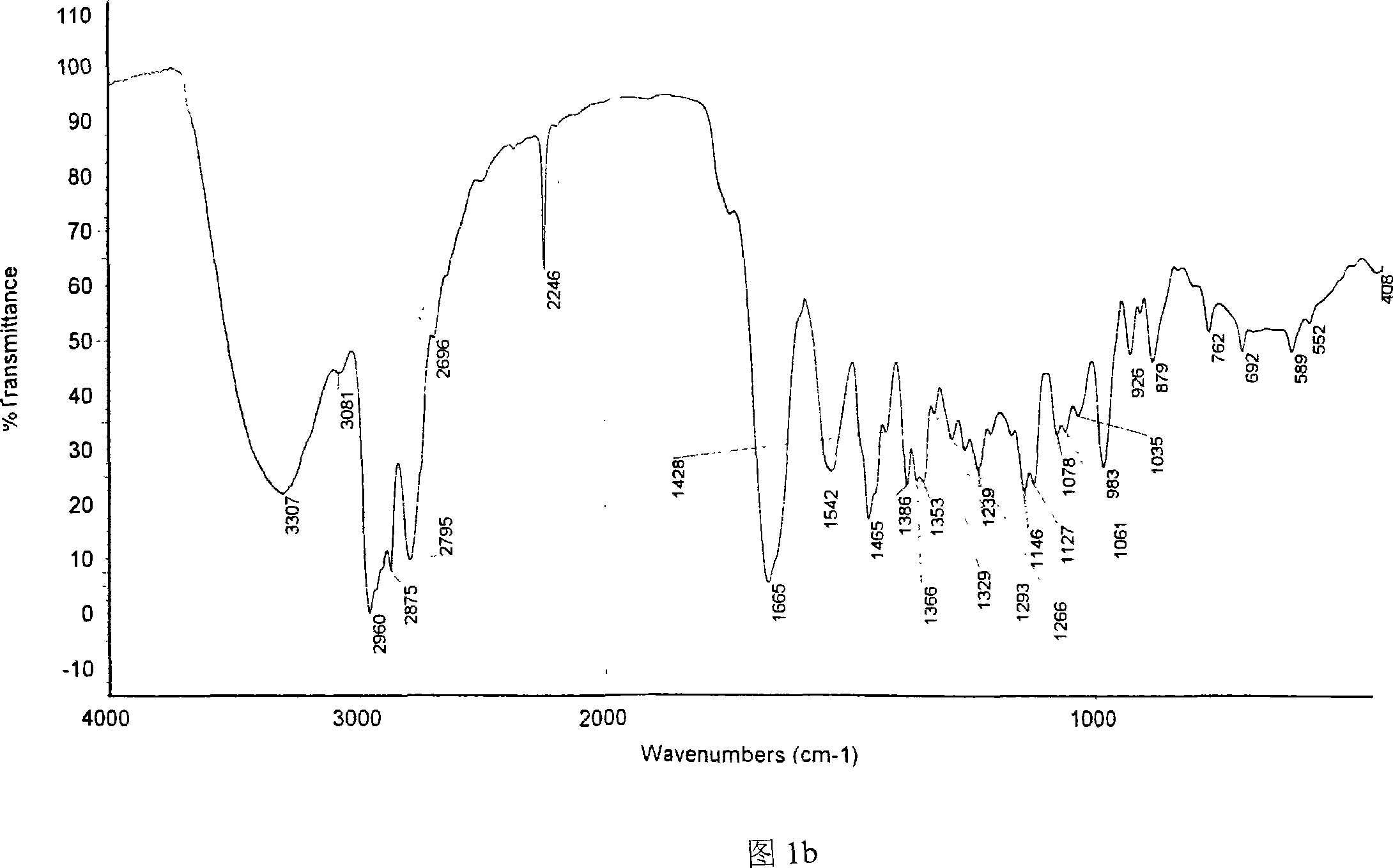
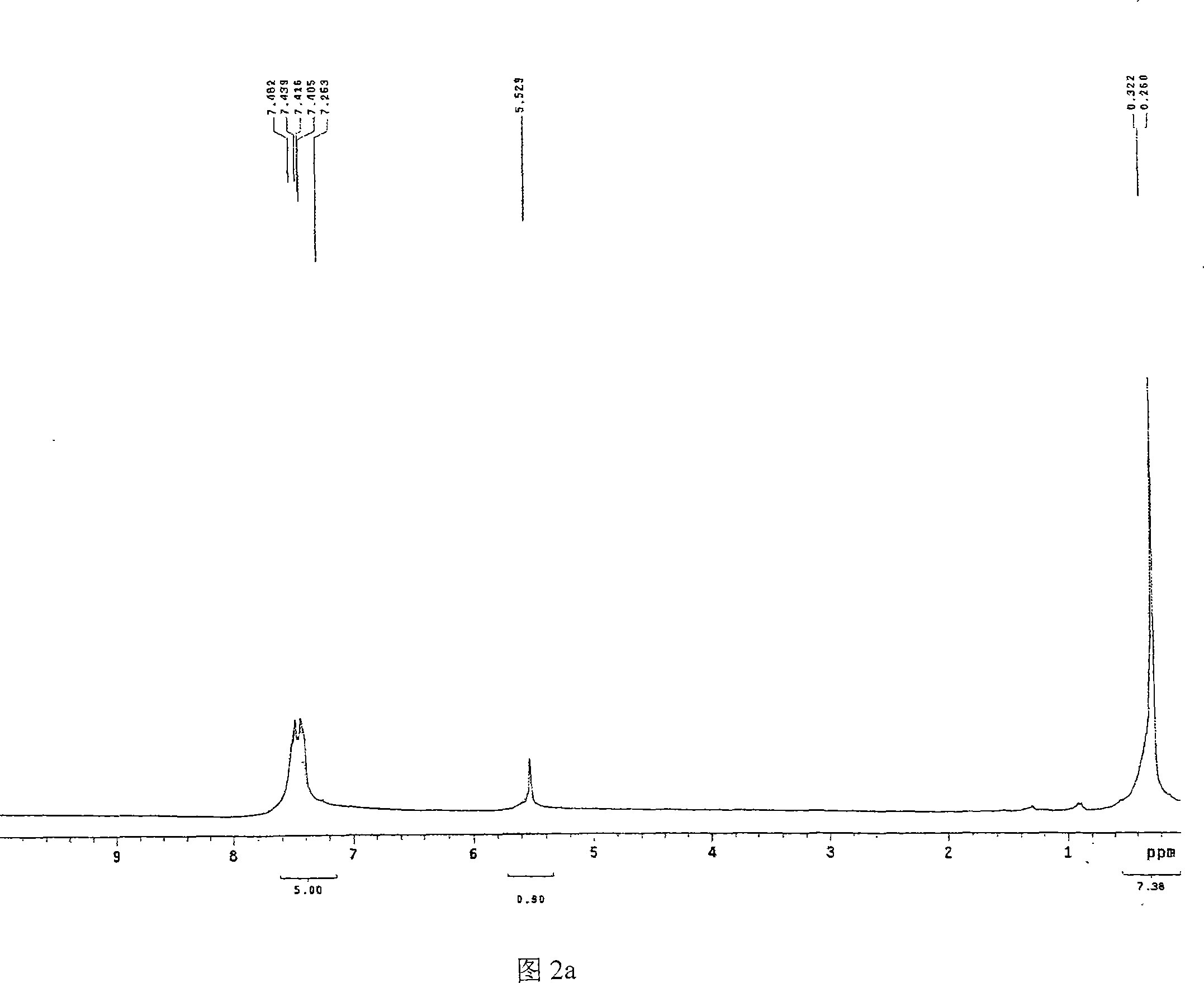
[0024] 1. Preparation of Catalysts 1a-1e
[0025] Now piperidine oxazoline, pyrrole oxazoline and ethyl zinc (ZnEt 2 ) as an example, non-limiting embodiments are described as follows:
[0026] When R is isobutyl, isopropyl, phenyl and benzyl, the catalyst prepared by piperidine oxazoline and ethyl zinc is respectively called catalyst 1a, 1b, 1c and 1d; pyrrole oxazoline and ethyl zinc are prepared The catalyst is called catalyst 1e.
[0027] 1a-1d: 1-[2-(4S)-4-isobutyl-4,5-dihydro-2-oxazolinyl-ethyl]piperidine, 1-[2-(4S)-4- Isopropyl-4,5-dihydro-2-oxazolinyl-ethyl]piperidine, 1-[2-(4S)-4-phenyl-4,5-dihydro-2-oxazoline Base-ethyl]piperidine, 1-[2-(4S)-4-benzyl-4,5-dihydro-2-oxazolinyl-ethyl]piperidine each 0.15g, respectively dissolved in THF solvent In, add ZnEt 2 0.4ml, reacted at room temperature for 24 hours, respectively prepared catalysts 1a-1d.
[0028] 1e: Preparation of 1-[2-(4S)-4-isopropyl-4,5-dihydro-2-oxazolinyl-ethyl]tetrahydropyrrole-zinc complex
[0029...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com