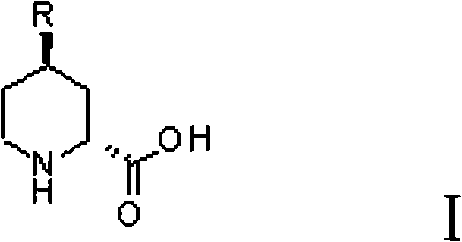
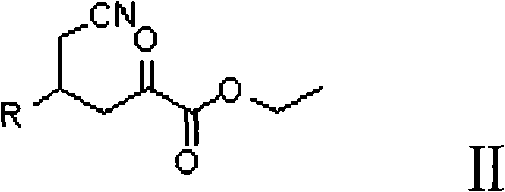
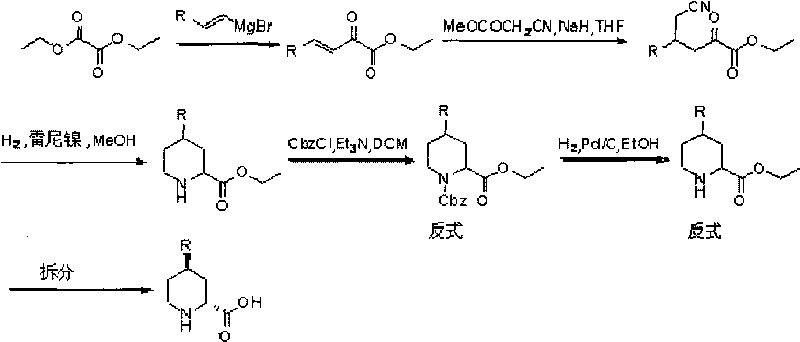
Preparation method for (2R, 4R)-4-substituted-2-piperidine carboxylic acid compound and intermediate thereof
A technology for piperidine carboxylic acid and compound, which is applied in the synthesis field of preparing -4-R-2-piperidine carboxylic acid compound, can solve the problems of expensive raw materials, complicated operation, complicated synthesis route and the like, and achieves wide application range, The effect of mild reaction conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (2R,4R)-4-Methyl-2-piperidinecarboxylic acid was prepared starting from diethyl oxalate and 1-bromo-propene.
[0032] (1) In a 100ml dry reaction flask, add 0.05mol of magnesium chips and 30ml of anhydrous tetrahydrofuran, and add 20ml of anhydrous tetrahydrofuran solution of 0.05mol of 1-bromo-propene into the addition funnel. First drop a little 1-bromo-propene solution into the reaction bottle, add a small grain of iodine, and heat slightly until the color disappears. At this time, the reaction has started, remove the heating, and start to add the tetrahydrofuran solution of 1-bromo-propene dropwise. After the dropwise addition, Continue to stir and react until the magnesium chips dissolve completely, and set aside. Add 0.05mol of diethyl oxalate and 80ml of anhydrous tetrahydrofuran to a dry 250ml reaction bottle, lower the temperature, protect with nitrogen, stir and slowly add the spare tetrahydrofuran solution above dropwise, after the dropwise addition, react fo...
Embodiment 2
[0040] (2R,4R)-4-phenyl-2-piperidinecarboxylic acid was prepared from diethyl oxalate and 1-bromo-3-phenyl-propene as starting materials.
[0041] Preparation method is with embodiment 1. total yield is about 28%
Embodiment 3
[0043] (2R,4R)-4-Methyl-2-piperidinecarboxylic acid was prepared starting from diethyl oxalate and 1-bromo-propene.
[0044] (1) In a 100ml dry reaction flask, add 0.05mol of magnesium chips and 30ml of ether, and add 20ml of 1-bromo-propene 0.05mol of ether solution into the addition funnel. First drop a little 1-bromo-propene solution into the reaction bottle, add a little dibromoethane, heat slightly until the reaction is triggered, remove the heat, start to drop the 1-bromo-propene tetrahydrofuran solution, after the dropwise addition, continue to stir the reaction until Magnesium chips are completely dissolved and ready for use. Add 0.05mol of diethyl oxalate and 80ml of ether to a dry 250ml reaction bottle, lower the temperature, protect with nitrogen, stir and slowly add the spare ether solution above. Ester was extracted, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness to obtain ethyl 2-carbonyl-3-enepentanoate. The yield of this step was abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com