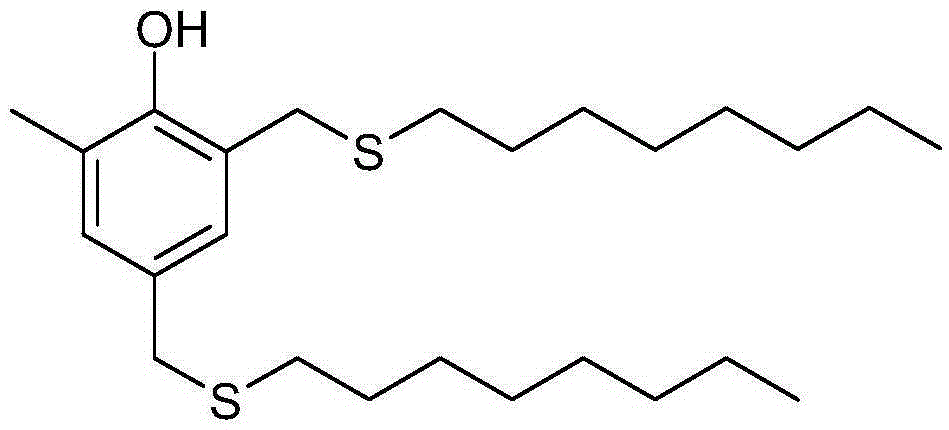
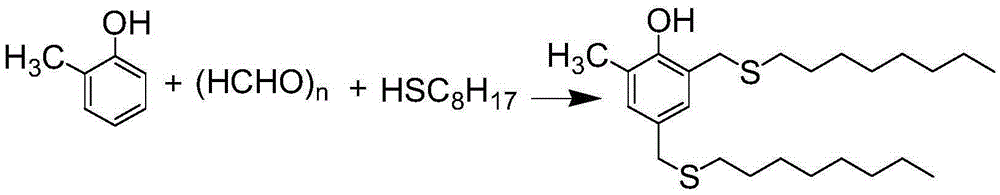
Preparation method for antioxidant 2,4-bi(n-octyl sulfur methylene)-6-methylphenol
An antioxidant, o-cresol technology, applied in the field of preparation of phenolic antioxidant 1520, can solve the problems of reducing product purity, limited application range, decolorization, etc., and achieves the effects of easy industrial production, simple process and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add paraformaldehyde (18.86g, 0.63mol), o-cresol (15.51g, 0.14mol), n-octyl mercaptan (45.64g, 0.31 mol), piperidine (3.11 g, 0.037 mol), solvent DMF (28.0 mL). Nitrogen protection gas was passed, and the oil bath was heated. First, the temperature was slowly raised to 80°C, and the reaction was performed for about 1 hour. After the reaction was completed, the solvent, water, catalyst and excess raw materials were removed by distillation under reduced pressure to obtain 59.78 g of crude product. Add a mixed solution of 60.0 mL of anhydrous methanol and 10 mL of toluene to the crude product, shake well, and carry out low-temperature stirring and crystallization. Suction filtration after the product is precipitated, the filter cake is washed with anhydrous methanol, and the solid product is collected, the filtrate continues to be crystallized at low temperature, and suction filtration is repeated 3 times, and most of the product is precipitated. The crystalline products...
Embodiment 2
[0033] Add paraformaldehyde (36.54g, 1.22mol), o-cresol (31.32g, 0.29mol), n-octyl mercaptan (87.98g, 0.63mol), piperidine (2.68g, 0.071mol), solvent DMF (60.0mL). Nitrogen protective gas was passed through, and the oil bath was heated. First slowly raise the temperature to about 90°C, react for about 0.5h, the solution becomes transparent, then raise the temperature to about 100°C, and react for 7h. The solvent, water, catalyst and excess raw materials were removed by distillation under reduced pressure, and 122.14 g of crude product was obtained by cooling. Add a mixed solution of 110mL of anhydrous methanol and 20mL of toluene to the crude product, shake well, stir and crystallize at low temperature. After the product was precipitated, it was suction filtered, and the filter cake was washed with anhydrous methanol to collect the solid product. The filtrate continued to crystallize at low temperature, and was filtered by suction, and so circulated 3 times, and most of the...
Embodiment 3
[0035]Add paraformaldehyde (54.60g, 1.82mol), o-cresol (48.56g, 0.45mol), n-octyl mercaptan (141.62g, 0.97mol), piperidine (8.03g, 0.09mol), solvent DMF (90.0mL). Nitrogen protection gas was passed, and the oil bath was heated. First, the temperature was slowly raised to 85°C, and the reaction was performed for about 1 hour. The solvent, water, catalyst and excess raw materials were removed by distillation under reduced pressure, and 192.10 g of crude product was obtained by cooling. Add a mixed solution of 170mL of anhydrous methanol and 30mL of toluene to the crude product, shake well, stir and crystallize at low temperature. After the product was precipitated, it was suction filtered, and the filter cake was washed with anhydrous methanol to collect the solid product. The filtrate continued to crystallize at low temperature, and was filtered by suction, and so circulated 3 times, and most of the products were precipitated. The crystalline products obtained three times we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com