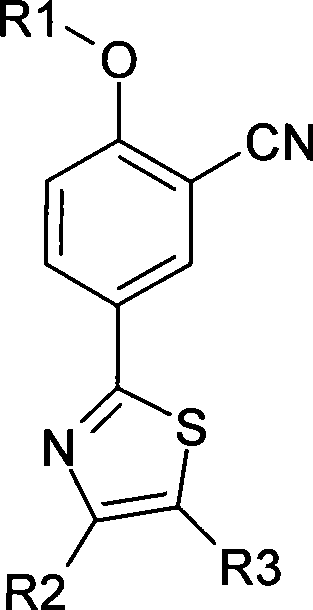
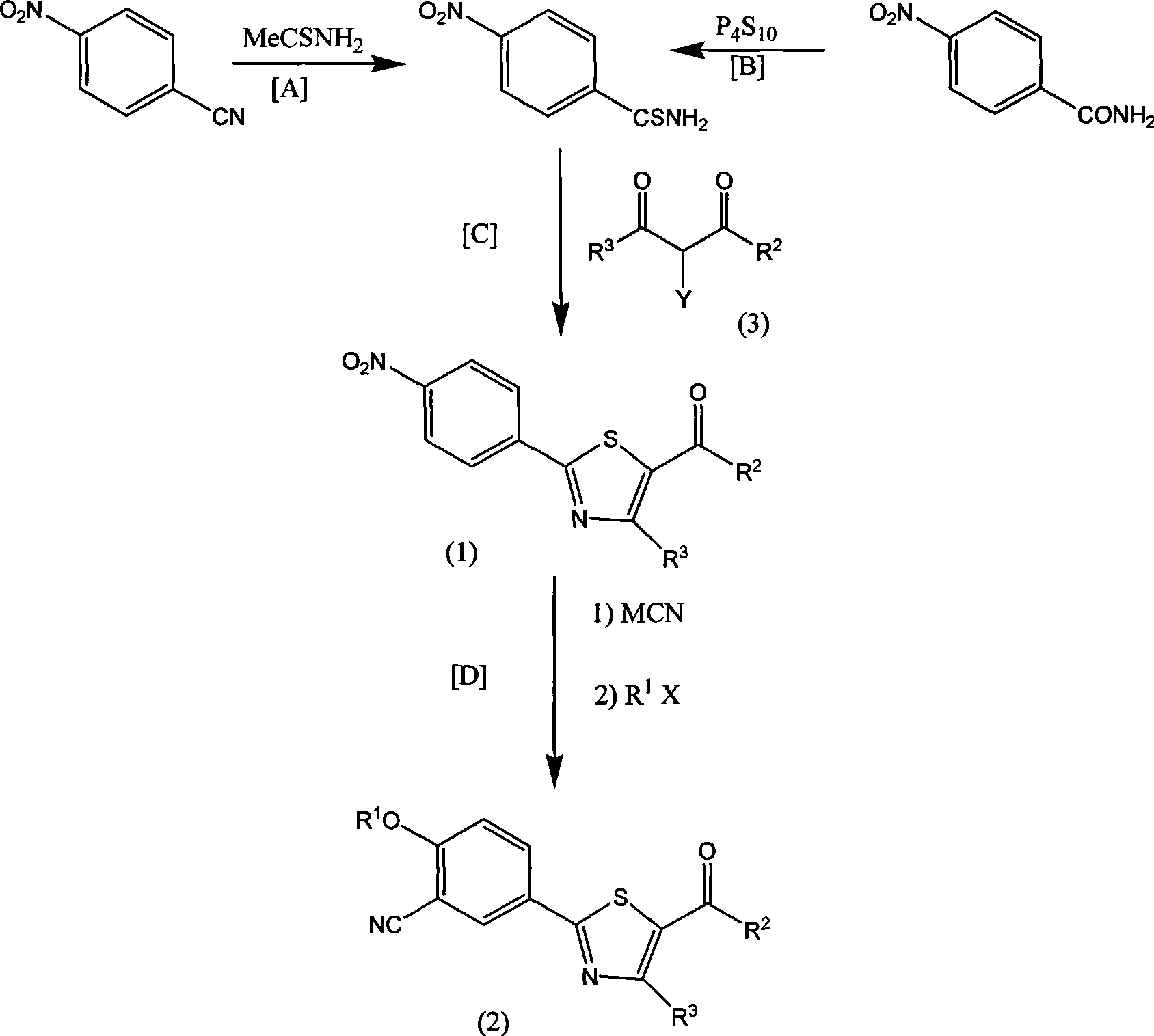
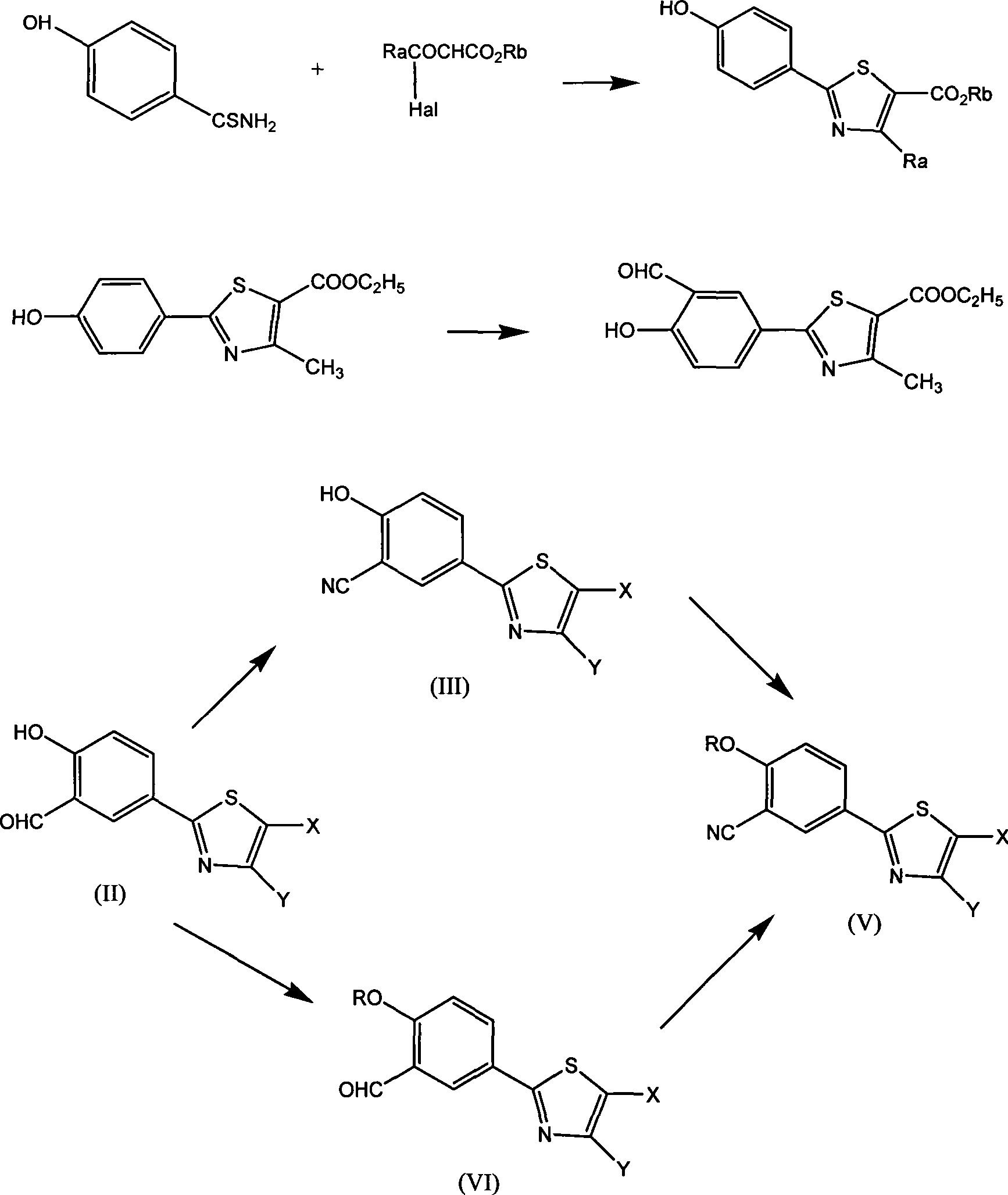
Aromatic nitrile-base thiazole derivatives for inhibiting xanthine oxidase activity, preparation method and application
A technology of xanthine oxidase and aromatic nitrile thiazole, which is applied in the directions of organic active ingredients, organic chemistry, drug combination, etc., can solve the problems of expensive raw materials, harsh operating conditions, and many reaction steps, and achieves simple operation and easy purification of products. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Ethyl 4-nitrobenzoate (19.5g, 0.1mol) and NaCN (4.9g, 0.1mol) were heated to 80°C in dry DMSO (50ml) and stirred for 3 hours. The reaction solution was cooled, and potassium carbonate ( 6.9g, 0.05mol) and isobutyl bromide (13.8g, 1mol), reacted at 60°C for 24 hours, poured the reaction solution into water, extracted with ethyl acetate, dried the organic layer with anhydrous sodium sulfate, concentrated to obtain an oil Product 4-isobutoxy-3-carbonitrile ethyl benzoate 1a, yield 83%. H1NMR (DMSO-D6): 1.02 (6H, d); 1.37 (3H, tri); 2.20 (H, m); 3.90 (2H, d); 4.27 (2H, m); 7.20 (1H, d); (H, dd); 8.15 (H, d); MS: m / z: 248 (M+1).
[0041]Ethyl 4-nitrobenzoate (19.5g, 0.1mol) and NaCN (7.35g, 0.15mol) were heated to 100°C in dry DMSO (500ml) and stirred for 1 hour. The reaction solution was cooled, and potassium carbonate ( 27.6g, 0.2mol) and isobutyl bromide (548g, 4mol), reacted at 150°C for 5 hours, recovered DMSO by rotary evaporation under reduced pressure, ...
Embodiment 2
[0049]
[0050] Add 2.47 grams of 4-isobutoxy-3-carbonitrile ethyl benzoate (0.01mol) and 5 ml of ethanol in the reaction flask, add 8% aqueous solution of 6 ml of sodium hydroxide, and hydrolyze at 30 C for 5 hours, and react The solution was neutralized with 0.5N hydrochloric acid solution, extracted by adding 10ml of ethyl acetate, washed with water, dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, added 1ml of anhydrous ether, 2ml of SOCl 2 , in CaCl 2 Under the protective device, after stirring at room temperature for 1 hour, add 0.1ml of DMF, heat to an internal temperature of 40°C, react for 2 hours, stop the reaction, the reaction solution is clear and transparent, concentrate under reduced pressure at 40°C, and after concentrating to near dryness, add 20ml CH 2 Cl 2 , after mixing, drop 5ml of CH in 5ml of concentrated ammonia water precooled to 0C 2 Cl 2 In the solution, 30 minutes after the dropwise reaction, TLC showed tha...
Embodiment 3
[0059]
[0060] Under the flow of dry nitrogen, add 60 g of phosphorus pentasulfide and 300 ml of phenetole in the reaction bottle and heat to reflux, react at 130 °C for 9 hours until all the solids are completely dissolved, accompanied by a large amount of H2S gas, use 10% NaOH Aqueous solution absorption. Stop heating when the H2S gas is no longer generated, and block crystals will form after cooling to room temperature. After the crystals were filtered and washed with ethyl acetate, they were vacuum-dried at room temperature to obtain 65 g of solid.
[0061] Under the flow of dry nitrogen, the above 8.5 grams of solids were added to a solution of 8.9 grams of 4-isobutoxy-3-carbonitrile benzamide in 50 mL of anhydrous THF, and after reacting at 50 C for 2 hours, The solvent THF was removed under reduced pressure, and the resulting residue was extracted with ethyl acetate. The organic layer was washed 3 times with water, dried over anhydrous sodium sulfate, and concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com