Methods respectively for producing optically active compound having agonistic activity on thrombopoietin receptors and intermediate of said compound
A compound and chemical formula technology, applied in the field of preparation of 1,3-thiazole derivatives, can solve problems such as not suitable for oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
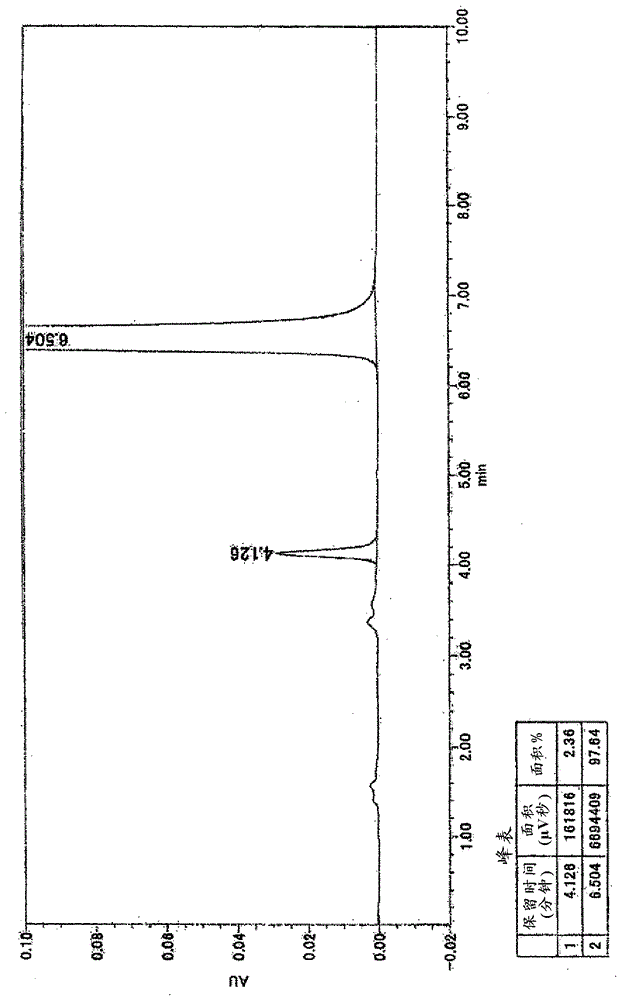
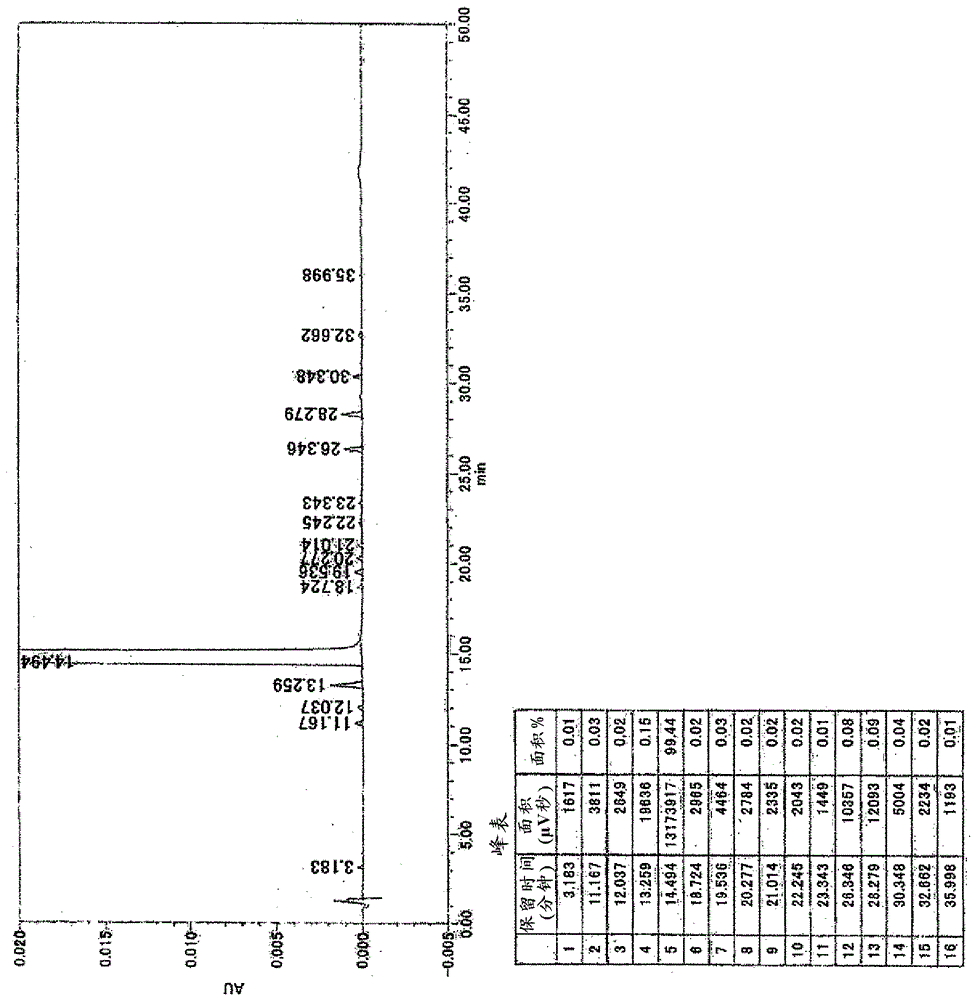
[0380] In terms of the crystals of compound (II'), crystals of compound (XI') and crystals of compound (VIII') or adducts thereof produced in the above-mentioned production method, stable, in performing the above-mentioned production steps or It is easy to handle in the preparation of a pharmaceutical composition containing compound (XI') as an active ingredient, and has high purity, so it is a useful crystal for the preparation of a pharmaceutical composition.
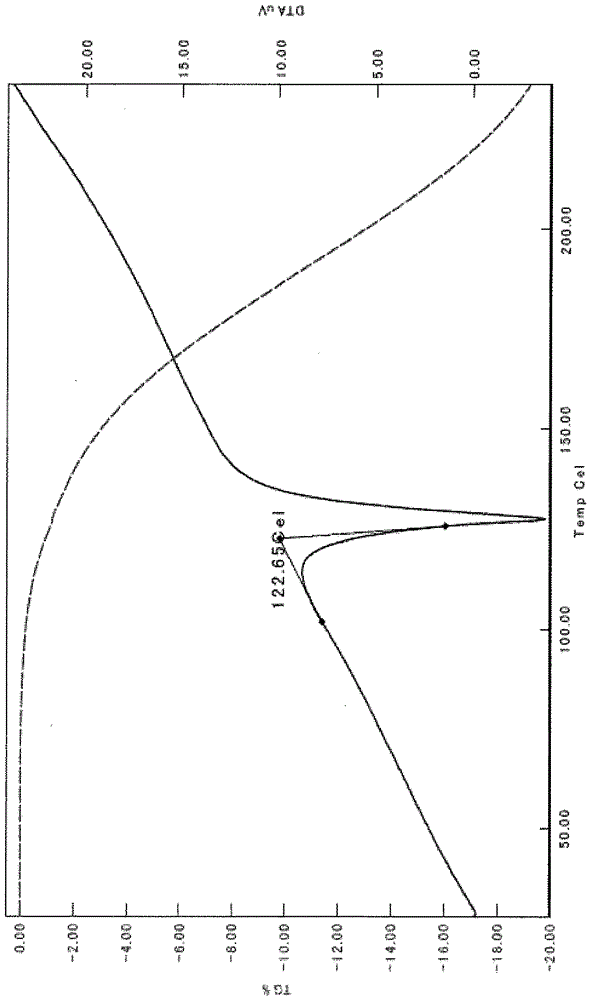
[0381] The crystals of the present invention can also be determined by thermal analysis.
[0382] Here, TG / DTA (simultaneous determination of differential calorimetry and thermogravimetry) is one of the main measurement methods of thermal analysis, and is a method of measuring the weight and thermal properties of a substance as an aggregate of atoms and molecules.
[0383] TG / DTA is a method of determining the temperature or time-dependent weight and heat changes of pharmaceutical active ingredients, by plotting the o...
Embodiment 1
[0448] [chemical formula 46]
[0449]
[0450] (Step 1) Synthesis of compound (VII')
[0451] Compound 1 (2.00 kg) was dissolved in 1,2-dimethoxyethane (28.0 kg) under nitrogen atmosphere. Thereto, a 25% LDA tetrahydrofuran-heptane-ethylbenzene solution (13.20 kg) was added dropwise at -55°C over 1 hour, followed by stirring for 30 minutes. A solution of N-formylmorpholine (3.74 kg) in 1,2-dimethoxyethane (3.0 kg) was added dropwise at -55°C over 40 minutes, followed by stirring for 1 hour. A 1,2-dimethoxyethane (3.0 kg) solution of triethyl 2-phosphonopropionate (3.74 kg) was dropped there over 45 minutes at 0° C., and stirred for 2 hours. A 35% sulfuric acid aqueous solution (15.8 kg) was added dropwise to the reaction liquid over 40 minutes. Water (16.0 kg) was added and extracted. The obtained organic layer was washed with water (8.0 kg), and the solvent was distilled off under reduced pressure. Acetonitrile (16.0 kg) was added, stirred at 25°C for 1 hour, cooled t...
Embodiment 2
[0460] (Synthesis of compound (XI'))
[0461]
[0462] (Step 2) Synthesis of compound 4
[0463] Compound 3 (3.00 kg) was added dropwise to a tetrahydrofuran solution (11.40 kg) of 1 mol / L isopropylmagnesium chloride over 1 hour at 25° C. under a nitrogen atmosphere, followed by stirring for 2 hours. At 25°C, a tetrahydrofuran solution (0.56 kg) of 1 mol / L isopropylmagnesium chloride was added, followed by stirring for 2 hours. At 25°C, N-methoxy-N-methylacetamide (1.45 kg) was added dropwise to the reaction liquid over 40 minutes, followed by stirring for 80 minutes. 7% hydrochloric acid (9.7 kg) was added to the reaction liquid, and extraction was performed with toluene (11.0 kg). The obtained organic layer was washed twice with water (7.5 kg each), and the solvent was distilled off under reduced pressure to obtain Compound 4 (2.63 kg).
[0464]
[0465] (Step 3) Synthesis of Compound 5
[0466] To compound 4 (2.63 kg) was added [(1S,2S)-N-(p-toluenesulfonyl)-1,2-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of incidence | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com