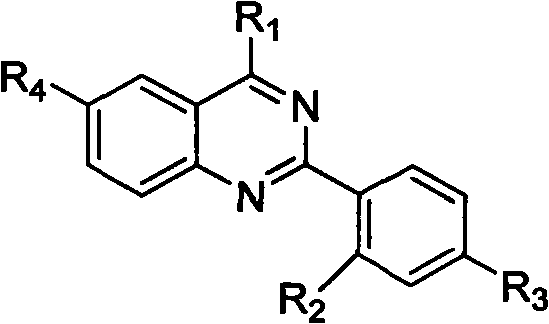
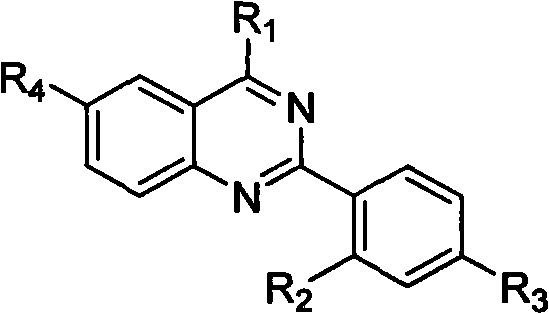
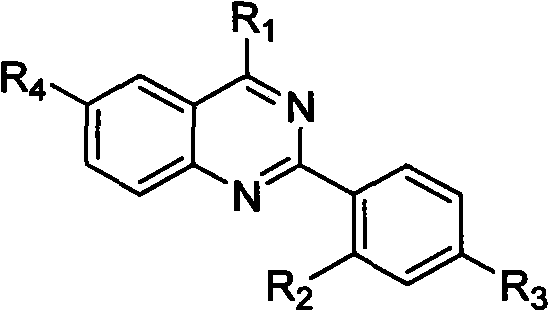
Quinazoline derivative and preparation method thereof and application of quinazoline derivative for preparing anticancer drugs
A technology of quinazoline and derivatives, which is applied in the field of preparing anticancer drugs, quinazoline derivatives and their preparation, can solve problems such as application limitations and limited resources, and achieve tumor suppression, increase groove binding force, The effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment one: the synthesis of compound QMC-2
[0038] Dissolve 549mmol of dry 4-chloro-2-nitrobenzoic acid in 50ml of thionyl chloride, and evaporate the thionyl chloride after reflux for 1.5h. The obtained brown liquid is slowly and dropwise added to the dissolved 766 mmol of anthranilamide and 1532 mmol of triethylamine in chloroform (200 ml), stirred at room temperature for 5 h, filtered, washed with ethanol, and recrystallized with ethanol to obtain white solid QMC-2.
[0039] Yield: 86%; 1 H NMR (400MHz, DMSO-d 6 ) 12.56(s, 1H), 8.44(d, J=8.2Hz, 1H), 8.39(s, 1H), 8.26(d, J=1.6Hz, 1H), 8.04-7.96(m, 1H), 7.93-7.85 (m, 2H), 7.81(s, 1H), 7.59(t, J=7.8Hz, 1H), 7.24(t, J=7.6Hz, 1H).ESI-MS m / z: 320[M+H] + .
[0040]
[0041] Compound QMC-2
Embodiment 2
[0042] Embodiment two: the synthesis of compound Q-2
[0043]
[0044] Compound Q-2
[0045] The method is the same as in Example 1, except that 2-nitrobenzoic acid is used instead of 4-chloro-2-nitrobenzoic acid to obtain white solid Q-2.
[0046] Yield: 88%; 1 H NMR (400MHz, DMSO) 12.57(s, 1H), 8.53(d, J=8.2Hz, 1H), 8.43(s, 1H), 8.13(d, J=8.3Hz, 1H), 7.95-7.78(m, 5H), 7.62(dd , J=11.4, 4.0Hz, 1H), 7.29-7.22(m, 1H).ESI-MS m / z: 286[M+H] + .
Embodiment 3
[0047] Embodiment three: the synthesis of compound QMC-3
[0048] Mix 376mmol dry QMC-2 with 100ml 10% potassium hydroxide aqueous solution and 100ml ethanol, and then react at 95°C for 4-5 hours. After finishing the reaction, distill off ethanol, adjust the pH value of the solution to between 1 and 3 with hydrochloric acid, and precipitate a large amount of white solid, filter and dry, and use petroleum ether / ethyl acetate (volume ratio 3 / 1) as eluent to pass through Purification by silica gel chromatography afforded QMC-3 as a white solid.
[0049] Yield: 92%; 1 H NMR (400MHz, DMSO) 12.87(s, 1H), 8.19(d, J=2.0Hz, 1H), 8.05(d, J=8.0Hz, 1H), 7.89(dd, J=8.4, 2.0Hz, 1H), 7.78(d, J =8.4Hz, 1H), 7.71(t, J=7.8Hz, 1H), 7.51(d, J=8.2Hz, 1H), 7.44(t, J=7.7Hz, 1H).ESI-MSm / z: 302 [M+H] + .
[0050]
[0051] Compound QMC-3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com