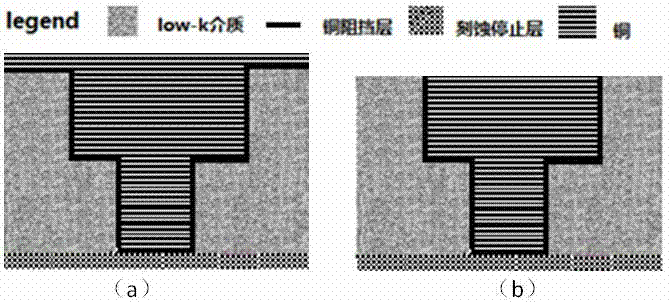
Polishing solution based on metal Co for polishing process
A polishing liquid and process technology, applied in the field of microelectronics technology, can solve the problems of cobalt copper desorption, fast polishing rate, copper butterfly pit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Polishing solution configuration: 5wt% silica sol; 0.5wt% hydrogen peroxide; 0.75wt% glycine (100 millimolar concentration); 0.06%-0.24wt% dimercaptothiazoline (5mM-20mM concentration); water. Adjust the pH value to 4.0;
[0017] Polishing equipment and mechanical parameter setting: The polishing machine used in this embodiment is the CP-4 table-top polishing machine produced by CETR Company; The rotational speed of the polishing table was 125 rpm.
[0018] The following is the comparison data of cobalt polishing rate with polishing fluid containing different dimercaptothiazoline concentrations.
[0019] Table 1: Polishing rate of cobalt in polishing liquid with different dimercaptothiazoline concentrations
[0020] Concentration of dimercaptothiazoline 0mM 5mM 10mM 15mM 20mM Cobalt polishing rate (nm / min) 905 153 122 119 87
[0021] It can be seen from the data in Table 1 that adding dimercaptothiazoline to the polishing solution c...
Embodiment 2
[0023] Polishing liquid configuration: 5wt% silica sol; 0.5wt% hydrogen peroxide; 0.75wt% glycine (100mM concentration); 0.18wt% dimercaptothiazoline (15mM concentration); and the balance of water. Adjust the pH value to 3.0, 4.0, 5.0 respectively;
[0024] Polishing equipment and mechanical parameter setting: The polishing machine used in this embodiment is the CP-4 table-top polishing machine produced by CETR Company; The rotational speed of the polishing table was 125 rpm.
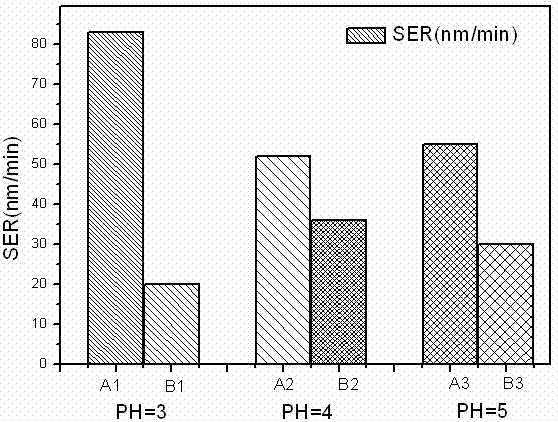
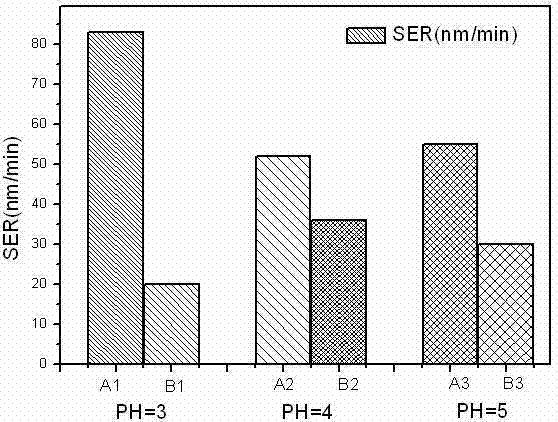
[0025] Table 2: Under different pH values, the polishing rate and selectivity ratio of copper and cobalt in the polishing solution containing and not containing dimercaptothiazoline
[0026]
[0027] From the data in Table 2, it can be clearly seen that after the addition of dimercaptothiazoline, the polishing rates of cobalt and copper decreased at various pH values. It shows that dimercaptothiazoline has inhibitory effect on cobalt and copper in the whole range of pH value 3-5. At PH value = 5, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com