Poly(allyl ether) compound, and preparation method and application thereof
A technology of polyallyl ether and hydroxyl compounds, applied in the fields of polymer chemistry and materials science, can solve the problems of rare reports of alkyne and hydroxyl monomers, achieve excellent regioselectivity and stereoselectivity, and simple process , the effect of high polymerization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
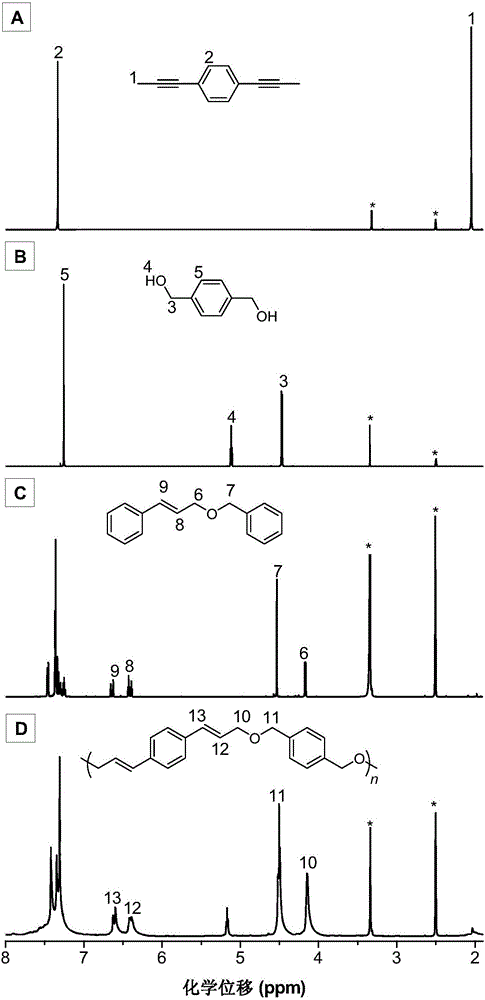
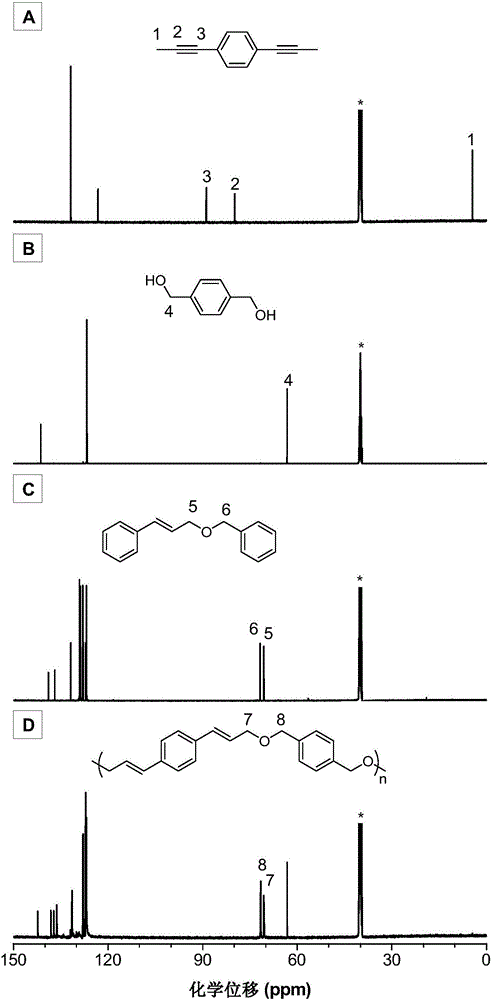
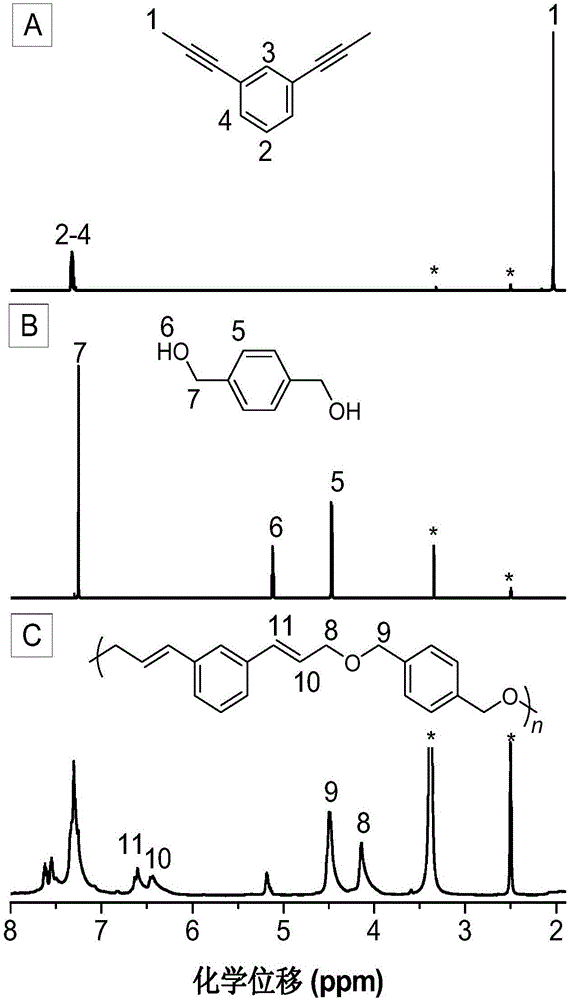
[0050]The synthetic method of monomer M1 in the present embodiment can be according to published literature (Unoh Y, Hirano K, SatohT, et al.An Approach to Benzophosphole Oxides through Silver-or Manganese-Mediated Dehydrogenative Annulation Involving C-C and C-P BondFormation.Angewandte Chemie International Edition, 2013, 52: 12975-12979.) synthetic method preparation; the specific process is as follows: 1,4-diethynylbenzene (1.26g, 10mmol) and a stirrer bar were added in a 100mL two-necked flask, vacuum exchanged Nitrogen gas 4 times, 15 min each time; add 30 mL of dry tetrahydrofuran, stir at -78°C for 0.5 h; add 2.5 M n-butyllithium n-hexane solution (30 mmol, 12 mL) dropwise, and continue stirring for 1 h after addition; methyl iodide (40mmol, 2.4mL) was added dropwise to the reaction system at 0°C, and naturally returned to room temperature to react overnight; the reaction was quenched with saturated ammonium chloride solution, the organic phase was collected by extractio...
Embodiment 2
[0062] The synthesis method of monomer M1 in this example is the same as that in Example 1; M3 is trans-1,6-cyclohexyldimethanol, which can be purchased from the market, and in this example was purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.
[0063] A preparation method of polyallyl ether compound (P2), the steps are as follows:
[0064] (1) Add 92.4mg (0.6mmol) of monomer M1, 86.4mg (0.6mmol) of monomer M3, 69.4mg (0.06mmol) of tetrakistriphenylphosphine palladium and 13.4mg (0.12mmol) of benzoic acid into a 25mL polymerization tube ), vacuumize and change nitrogen for 3-5 times, inject 1.0mL of 1,4-dioxane with a syringe; place the polymerization tube in an oil bath at 100°C and stir for 24 hours (rotation speed: 400 rpm), After the reaction, the reaction solution was diluted to 30 mL with dichloromethane, extracted with 100 mL*3 of saturated saline, the organic phase was combined, and the organic phase was concentrated to 5 mL;
[0065] (2) Under the...
Embodiment 3
[0070] The synthesis method of monomer M1 in this example is the same as that in Example 1; M4 is 1,6-hexanediol, which can be purchased from the market, and in this example was purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd.
[0071] A preparation method of polyallyl ether compound (P3), the steps are as follows:
[0072] (1) Add 92.4mg (0.6mmol) of monomer M1, 70.8mg (0.6mmol) of monomer M3, 69.4mg (0.06mmol) of tetrakistriphenylphosphine palladium and 13.4mg (0.12mmol) of benzoic acid into a 25mL polymerization tube ), evacuate and change nitrogen for 3 times, inject 1.0mL of 1,4-dioxane with a syringe; place the polymerization tube in an oil bath at 100°C and stir for 24 hours (rotation speed: 400 rpm), and the reaction ends Finally, dilute the reaction liquid with dichloromethane to 30 mL, extract with 100 mL of saturated brine, combine the organic phases, and concentrate the organic phases to 5 mL;
[0073] (2) Under the condition of stirring (the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com