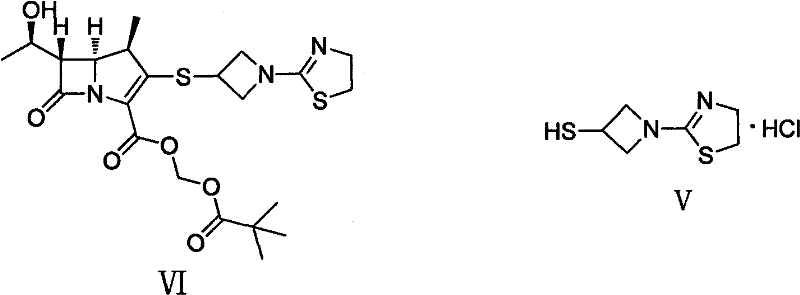
Preparation method of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride
A technology based on mercaptoazetidine hydrochloride and thiazoline, applied in the new preparation field of 1--3-mercaptoazetidine hydrochloride, can solve the problems of long route, harsh reaction conditions, high cost, etc. The route is simple, the raw materials are easy to obtain, and the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
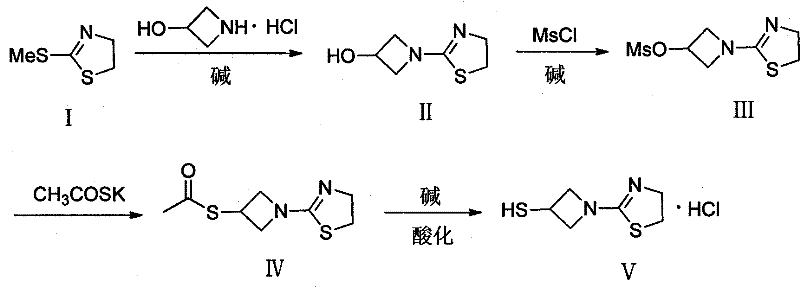
[0032] The first step: the preparation of 1-(4,5-dihydro-2-thiazolinyl)-3-hydroxyazetidin (II)
[0033] Add 3-hydroxyazetidine hydrochloride (103.68g) in dry there-necked flask, KHCO 3 (124.50g) and methanol, stirred at room temperature, then added 2-methylthio-2-thiazoline (120.00g), reacted at 80°C for 10h, cooled to room temperature, filtered, the filtrate was concentrated, and chloroform (600ml) was added to the residue , the filtered solid was dissolved in water (600ml), the two were mixed and then separated, the aqueous phase was extracted with chloroform, the combined filtrate was washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to obtain a white solid, and dried in vacuo to obtain intermediate 94.31 g of 1-(4,5-dihydro-2-thiazolinyl)-3-hydroxyazetidin (II), with a yield of 66%, was directly put into the next reaction.
[0034] 1 H NMR (400MHz, CDCl 3 )δ: 7.28(s, 1H), 4.67-4.61(m, 1H), 4.18(dd, J=6.88, 8.20Hz, 2...
Embodiment 2
[0036] The first step: the preparation of 1-(4,5-dihydro-2-thiazolinyl)-3-hydroxyazetidin (II)
[0037] In dry there-necked flask, add 3-hydroxyazetidine hydrochloride (108.64g), K 2 C0 3 (137.00g) and ethanol, stirred at room temperature, then added 2-methylthio-2-thiazoline (120.00g), reacted and refluxed at 100°C for 8h, cooled to room temperature, filtered, the filtrate was concentrated, and chloroform (600ml ), the filtered solid was dissolved in water (600ml), the two were mixed and then separated, the aqueous phase was extracted with chloroform, the combined filtrate was washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to obtain a white solid, and dried in vacuo 131.55 g of the intermediate 1-(4,5-dihydro-2-thiazolinyl)-3-hydroxyazetidin (II) was obtained with a yield of 92%.
Embodiment 3
[0039] The second step: the preparation of 1-(4,5-dihydro-2-thiazolinyl)-3-methanesulfonate group azetidine (III)
[0040] Get 1-(4,5-dihydro-2-thiazolinyl)-3-hydroxy azetidin (II) (40.00g) and Et 3 N (38.38g) was added into dichloromethane (250ml), MsCl (37.69g) was added dropwise at 0°C, then stirred at the same temperature for 8h, filtered, the filtrate was washed with saturated sodium bicarbonate solution, and the aqueous phase was reused Back extraction with dichloromethane, the combined filtrate was washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, recrystallized in a mixed solution of ethyl acetate and n-hexane (10:1), filtered the solid, washed, and dried in vacuo to obtain White solid 39.07g, yield 66%.
[0041] 1 H NMR (400MHz, CDCl 3 )δ: 5.31-5.28(m, 1H), 4.37-4.32(m, 2H), 4.19-4.15(m, 2H), 4.03(t, J=7.56, 2H), 3.39(t, J=7.52Hz, 2H), 3.06(s, 3H);
[0042] MS(EI):m / z[M] + calcd for C 7 h 12 N 2 o 3 S ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com