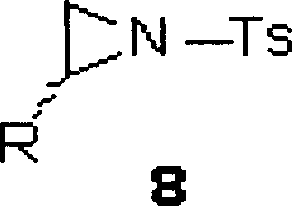Chirality sulfonamide aminol ligand based on prolinol, its preparing method and application
A technology for sulfonamidoamine alcohol and prolinol, which is applied in the field of chiral sulfonamidoamine alcohol ligands, can solve the problems of low overall yield and the like, and achieve the effects of high reaction yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
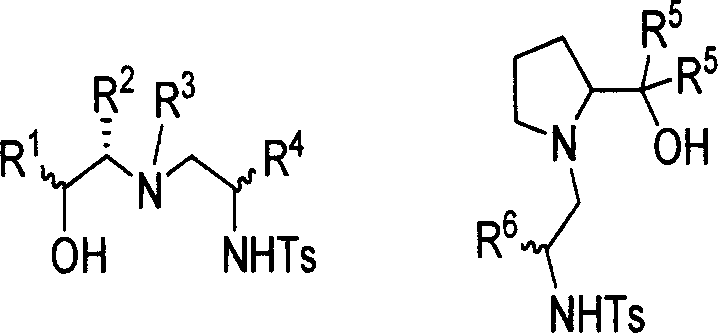
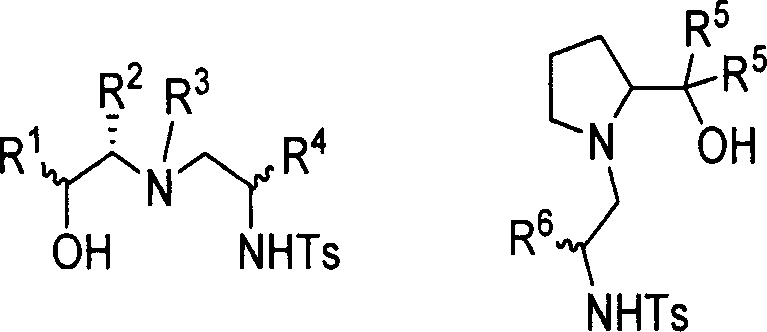
[0034] Commercially available L-prolinol (3.80mmol, 0.39g) and (R)-2-phenyl-1-tosylcycloethyleneimine (3.66mmol, 1.00g) were dissolved in 30ml of acetonitrile The mixture was kept under reflux for 10 hours, and TLC detection found that the reaction had basically ended. The solvent was distilled off under reduced pressure, and the residue was recrystallized from a mixed solvent of dichloromethane and petroleum ether (60-90°C) to obtain a white solid (0.89g), with a yield of 65% and a melting point of 113 -114℃; Specific rotation is [α] D 25 =+1.33(c0.5, CHCl 3 ); NMR data are: 1 H NMR (CDCl 3 ): 1.61-1.69(m, 4H), 2.44(s, 3H), 2.62(d, J=6.8Hz, 1H), 2.87(t, J=8.0 Hz, 2H), 3.03-3.06(m, 2H ), 3.17-3.22(m, 1H), 3.49-3.54(m, 1H), 3.63-3.66(m, 1H), 7.14-7.16(m, 2H), 7.29(t, J=5.2Hz, 5H), 7.68(d, J=8.0Hz, 2H); 13 C NMR (CDCl 3 ): 21.71, 24.51, 29.10, 45.68, 52.77, 61.54, 64.59, 66.56, 127.28, 128.58, 129.01, 129.88, 137.02, 138.94, 143.64; high-resolution mass spectrometry da...
Embodiment 2
[0036] Apply the ligand synthesized in Example 2 to the asymmetric addition reaction of diethylzinc to α-naphthaldehyde, use n-hexane as solvent, stir at room temperature for about 20 hours, and obtain the product with absolute configuration S , the chemical yield is 90%, and the enantioselectivity exceeds 99%.
Embodiment 3
[0038] Apply the ligand synthesized in Example 2 to the asymmetric addition reaction of diethylzinc to o-chlorobenzaldehyde, use n-hexane as solvent, stir at room temperature for about 20 hours, and obtain the product with absolute configuration R , the chemical yield is 92%, and the enantioselectivity exceeds 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com