Polyimidazole compound, in-situ preparation method and applications thereof
An in-situ preparation and compound technology, which is applied in the field of polyimidazole compounds and their in-situ preparation, can solve the problems of limited polymerization performance, restricted development of polyimidazole compounds, etc., and achieves high polymerization efficiency, excellent stereoselectivity, and operation. convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
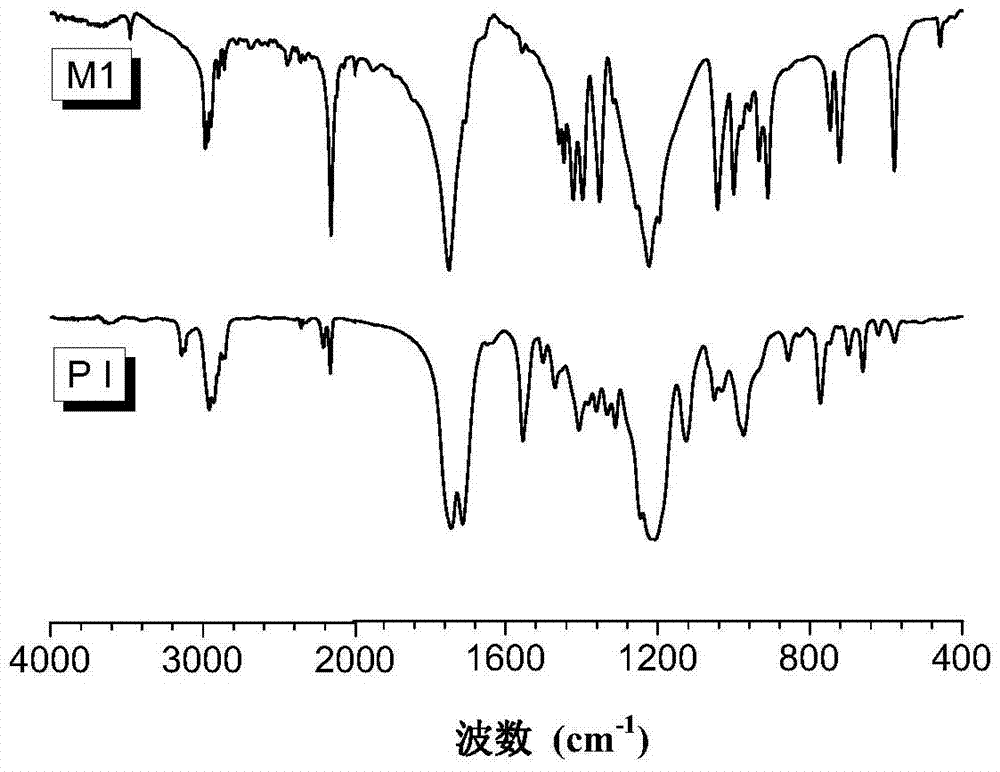
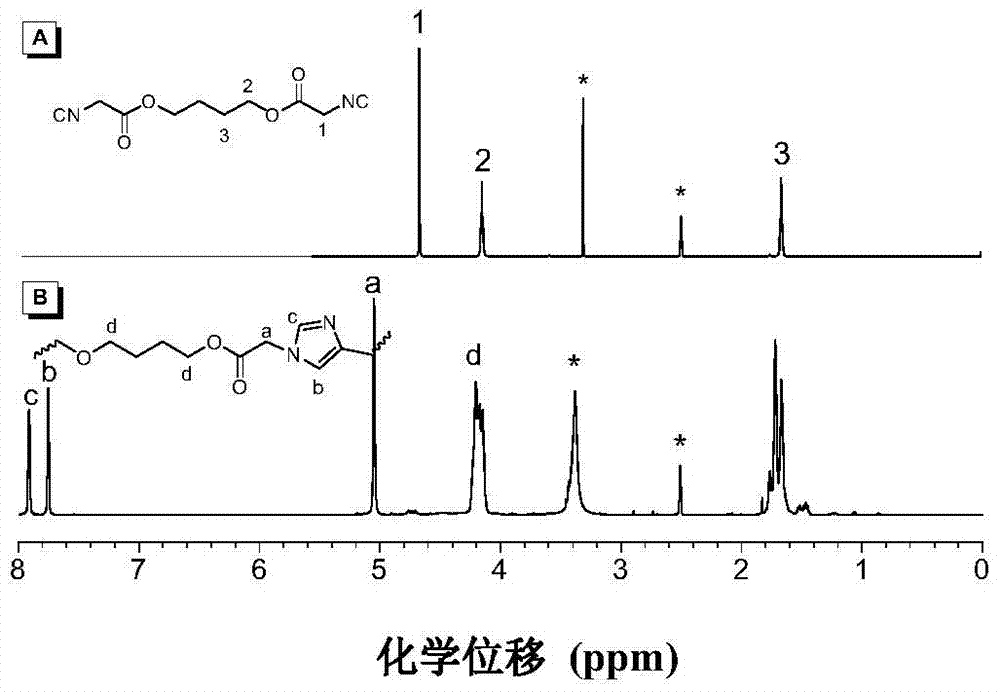
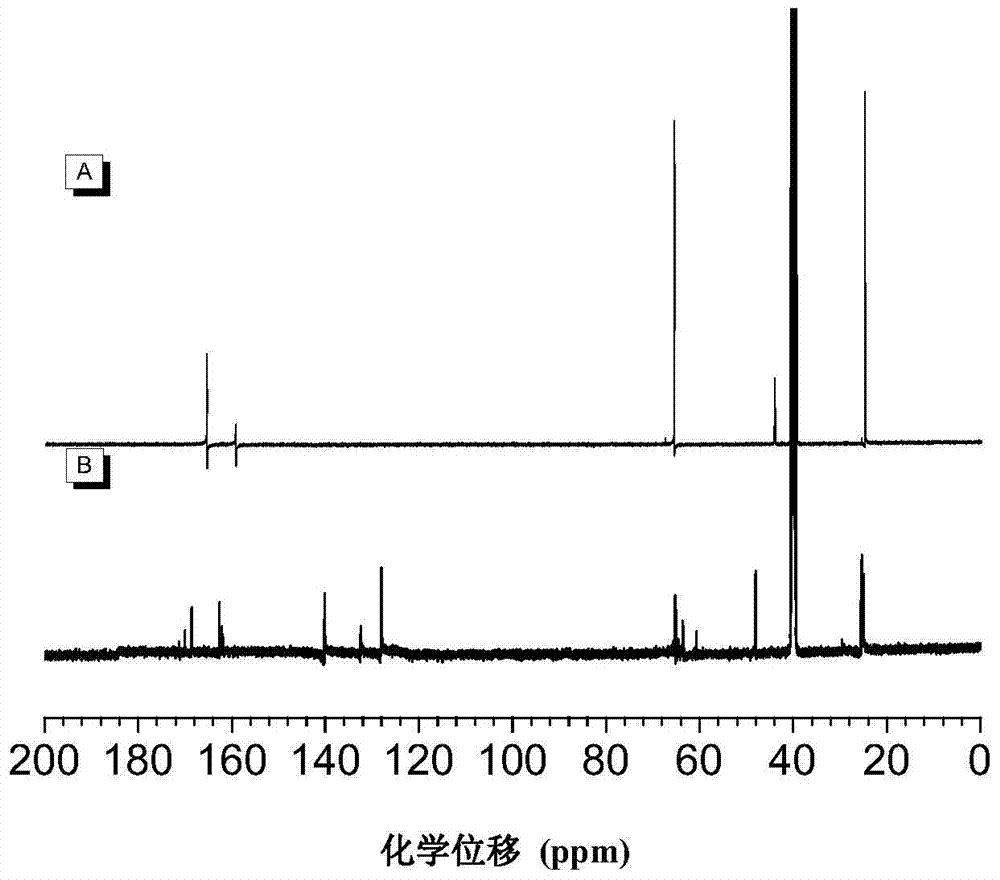
[0077] The polyimidazole compound of preparation structural formula as shown in PII:
[0078]
[0079] 1) Firstly, monomer 3 was synthesized through reaction formula (1), that is, the above-mentioned monomer wherein R is (V-2).
[0080]
[0081] Formula (1)
[0082] The specific preparation process of the monomer 3 is as follows:
[0083] (1) Under the protection of inert gas, compound 1 (potassium isocyanoacetate) and compound 2 (1,6-dibromohexane) were mixed at a ratio of 1:0.45, and fully reacted in DMF solvent at 50°C for 8 hours .
[0084] (2) After the reaction, the reaction solvent DMF was removed, and the final product was separated by column chromatography using a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 1:1 as the eluent to obtain monomer 3.
[0085] Utilize monomer 3 to prepare polymer PII by reaction formula (two), concrete steps are as follows:
[0086] 1) Under the protection of an inert gas, add the monomer 3 and the ...
Embodiment 2~5
[0097] Embodiment 2~5 investigated the influence of different reaction temperatures on reaction conditions. Wherein, the preparation of polymerized monomers and the steps of using monomers to prepare polymers are the same as in Example 1, the difference lies in the polymerization reaction temperature. The specific polymerization conditions are shown in Table 1.
[0098] Table 1. Effect of polymerization reaction temperature on the polymerization of monomer 3 a
[0099]
[0100] a Reaction in acetonitrile in air; reaction time 2h; [M] 0 = 0.5M.
[0101] b T = reaction temperature.
[0102] c Determined by GPC with linear polystyrene as the calibration substance and DMF as the mobile phase.
[0103] It is completely soluble in common organic solvents such as DMF (dimethylformamide) and DMSO (dimethyl sulfoxide).
[0104] It can be seen from Table 1 that the polymerization activity is very high, and polymers with relatively high molecular weight can also be generated...
Embodiment 6~8
[0106] Examples 6-8 investigated the influence of different monomer concentrations on the reaction conditions. The preparation of polymerized monomers and the steps of using monomers to prepare polymers are the same as in Example 1, except that the concentration of monomers in the polymerization is different. , the specific reaction conditions and results are shown in Table 2.
[0107] Table 2. Effect of monomer concentration on polymerization of monomer 3 a
[0108]
[0109] a Reaction in acetonitrile in air; reaction time 2h; temperature at room temperature (25°C).
[0110] b Determined by GPC with linear polystyrene as the calibration substance and DMF as the mobile phase.
[0111] It is completely soluble in common organic solvents such as DMF and DMSO.
[0112] It can be seen from Table 2 that too high or too low monomer concentration is not conducive to the occurrence of polymerization reaction, and too low concentration will cause the probability of collision be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com