Preparation method of 2,5-dichlorophenol
A technology of dichlorophenol and dichlorobenzene is applied in the field of manufacture of 2, pesticides and pharmaceutical intermediates, and achieves the effects of few operation steps, mild conditions and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
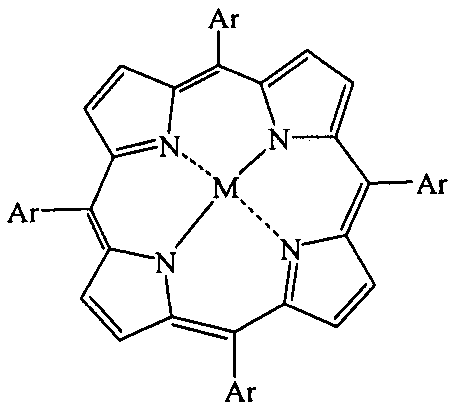
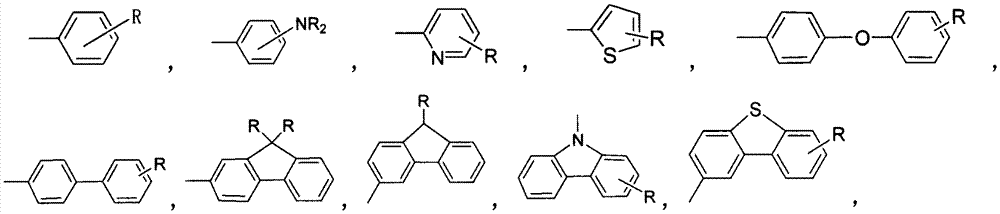
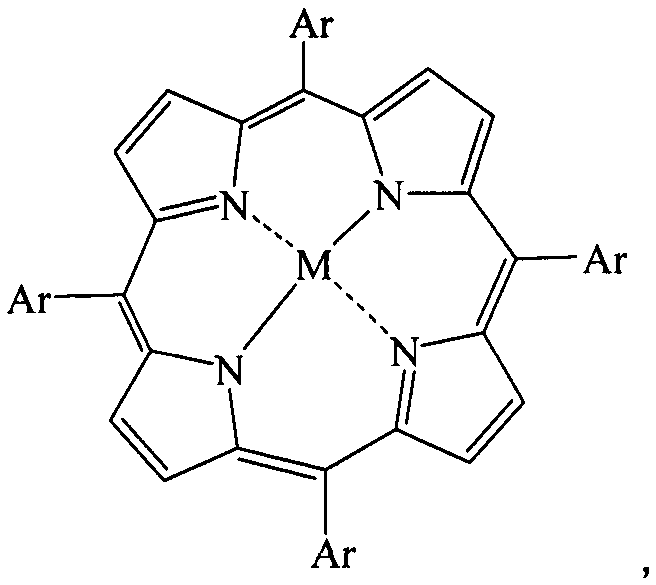
[0027] In a 1000mL four-necked reaction flask, sequentially add 14.7g 1,4-dichlorobenzene, 100mL acetonitrile, vanadium porphyrin compound (M=vanadium, Ar=phenyl, R=H), 0.5g cocatalyst phosphoric acid, slowly drop The oxidizing agent was 5 mL of 30% hydrogen peroxide, the reaction temperature was controlled at 20° C., and the reaction was carried out for 6 hours. After the reaction was completed, the reaction solution was filtered to remove insoluble matter, and the conversion rate of 1,4-dichlorobenzene was 98.0% and the selectivity of 2,5-dichlorophenol was 95.3% through quantitative analysis by high performance liquid chromatography.
Embodiment example 2
[0029] In a 1000mL four-necked reaction flask, add 14.7g1,4-dichlorobenzene, 100mL acetic acid, 0.02g vanadium porphyrin compound (M=vanadium, Ar=phenyl, R=H), 0.5g cocatalyst phosphoric acid, slowly 5 mL of 30% hydrogen peroxide was added dropwise, the reaction temperature was controlled at 20° C., and the reaction was carried out for 6 h. After the reaction, the reaction liquid was filtered to remove insoluble matter, and the conversion rate of 1,4-dichlorobenzene was 85.0% and the selectivity of 2,5-dichlorophenol was 92.3% through quantitative analysis by high performance liquid chromatography.
Embodiment example 3
[0031] In a 1000mL four-necked reaction flask, add 14.7g1,4-dichlorobenzene, 100mL methanol, 0.02g vanadium porphyrin compound (M=vanadium, Ar=phenyl, R=H), 0.5g cocatalyst phosphoric acid, slowly 5 mL of 30% hydrogen peroxide was added dropwise, the reaction temperature was controlled at 20° C., and the reaction was carried out for 6 h. After the reaction, the reaction solution was filtered to remove insoluble matter, and quantitative analysis by high performance liquid chromatography showed that the conversion rate of 1,4-dichlorobenzene was 75.0%, and the selectivity of 2,5-dichlorophenol was 96.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com