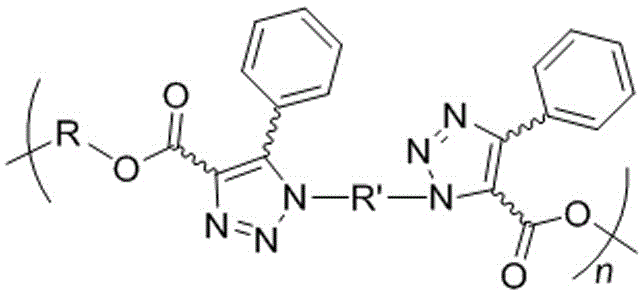
Polyphenyltriazole formate, and preparation method and application thereof
A technology of polyphenyltriazole formate and phenylpropiolic acid dibasic ester, which is applied in the field of polymer chemistry and material science, can solve the problem of cytotoxicity affecting the photoelectric performance of polymers, polytriazole with poor solubility and difficult to remove. and other issues, to achieve the effects of wide substrate applicability, good functional group compatibility, and prevention of explosive attacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
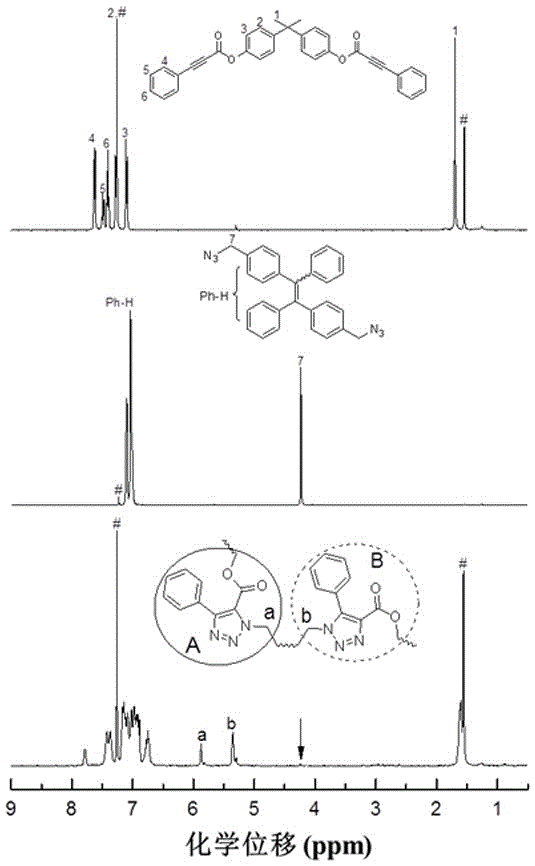
[0040]Add 1.14 g (5 mmol) of bisphenol A, 3.10 g (15 mmol) of DCC, 0.244 g (2 mmol) of DMAP, and 0.38 g (2 mmol) of TsOH into a 250 mL two-necked flask, vacuumize and fill with nitrogen three times. Add 100 mL of dichloromethane and stir to dissolve, then dissolve 1.61 g (11 mmol) phenylpropylic acid in 20 mL of dichloromethane under an ice-water bath environment, and add it dropwise to the reaction system through a constant pressure dropping funnel. The reaction was stirred overnight at room temperature, filtered, washed with dichloromethane, and the filtrate was spin-dried to obtain a crude product, which was separated and purified by column chromatography, dried in vacuo to constant weight, and 1.89 g of a white solid (yield 78.1% ), which is the first monomer phenylpropiolic acid dibasic ester monomer. 1 H NMR (400 MHz, DMSO- d 6 ), δ (TMS,ppm): 7.71 (d, J = 7.4 Hz, 4H), 7.62 (t, J = 7.4 Hz, 2H), 7.52 (t, J = 7.5Hz, 4H), 7.33 (d, J = 8.6 Hz, 4H), 7...
Embodiment 2
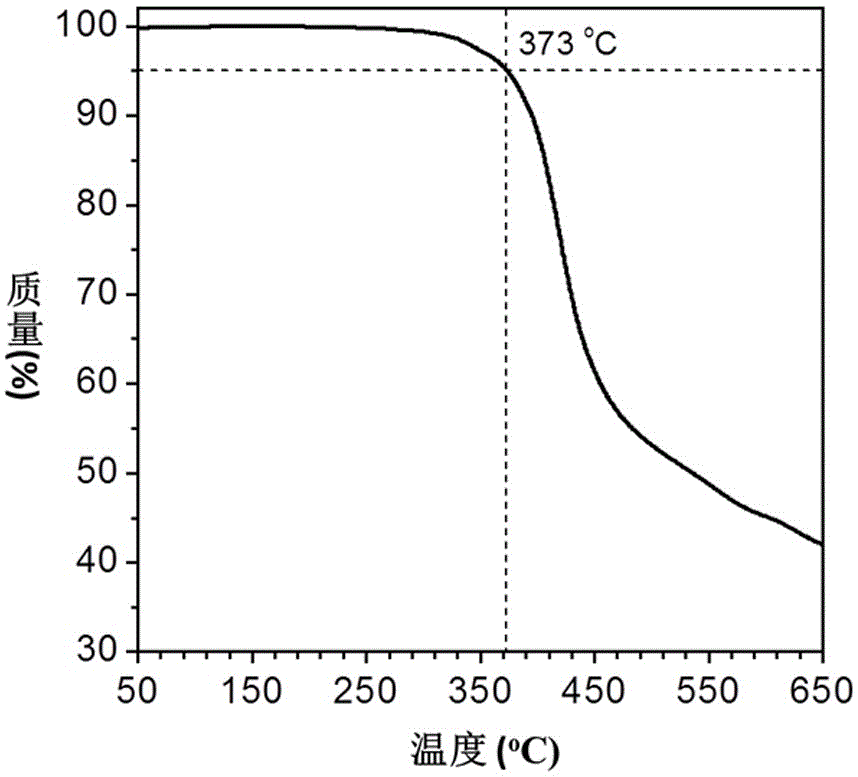
[0048] The phenylpropiolic acid dibasic ester monomer and the binary organic azide monomer are the same as in Example 1. Add 48.4 mg (0.1 mmol) of the first monomer and 44.2 mg (0.1 mmol) of the second monomer into a 10 mL polymerization tube, vacuumize the system and fill with nitrogen, repeat three times, then add 0.2 mL of dry DMF, and wait for a single After the body is completely dissolved, react at 150°C for 1 hour, cool to room temperature and dilute with 5 mL of chloroform, and drop the solution into 200 mL of vigorously stirred n-hexane through a dropper plugged with cotton, let stand, filter, Dry to constant weight to obtain a polymer with a yield of 85.1%. GPC results show: M w = 10100, PDI = 1.4. The polymer also has good solubility and thermal stability; due to the tetraphenylethylene group containing aggregation-induced luminescence activity, the polymer also has aggregation-induced luminescence properties and can be used for the detection of explosives, as ...
Embodiment 3
[0050] The phenylpropiolic acid dibasic ester monomer and the binary organic azide monomer are the same as in Example 1. Add 48.4 mg (0.1 mmol) of the first monomer and 44.2 mg (0.1 mmol) of the second monomer into a 10 mL polymerization tube, vacuumize the system and fill with nitrogen, repeat three times, then add 0.2 mL of dry DMF, and wait for a single After the body is completely dissolved, react at 150°C for 4 hours, cool to room temperature and dilute with 5 mL of chloroform, and add the solution dropwise into 200 mL of vigorously stirred n-hexane through a dropper plugged with cotton, let stand, and filter , and dried to constant weight to obtain a polymer with a yield of 78.3%. GPC results show: M w = 24800, PDI = 1.8. The polymer also has good solubility and thermal stability; due to the tetraphenylethylene group containing aggregation-induced luminescence activity, the polymer also has aggregation-induced luminescence properties and can be used for the detectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com