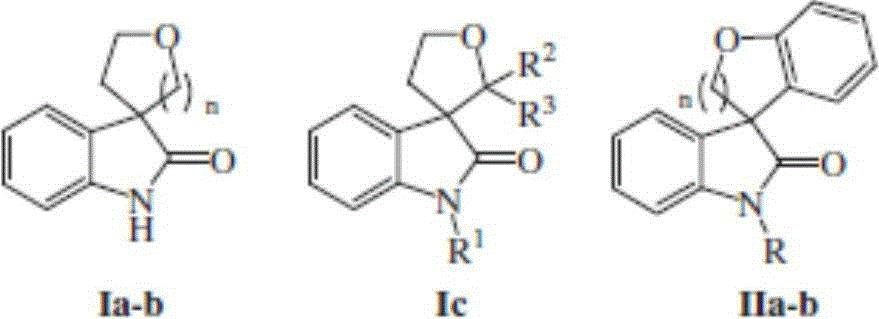
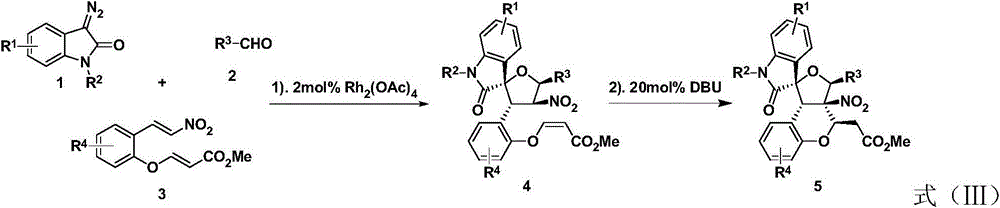
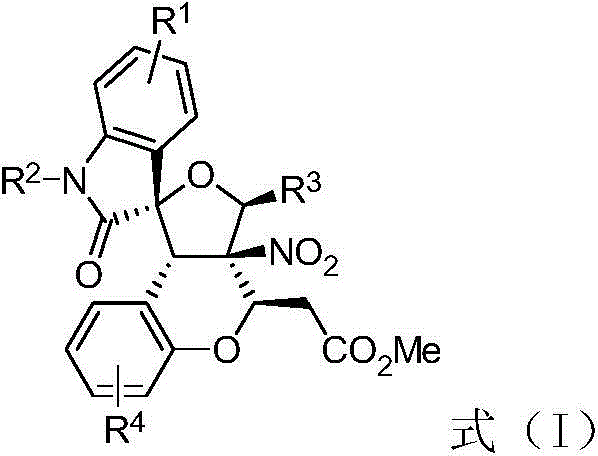
Preparation method of 3, 3-spiro (2-tetrahydrofuranyl)-oxindole polycyclic compound
A technology of cyclic compounds and tetrahydrofuran, which is applied in 3 fields, can solve the problems of low substrate universality and harsh reaction conditions, and achieve the effects of low synthesis cost, short preparation route and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 prepares compound 5a of the present invention:
[0027] Weigh 5-chlorophenylnitroalkene 3a (0.20mmol), rhodium acetate (1.70mg, 0.004mmol), p-bromobenzaldehyde 2a (0.30mmol), Molecular sieves (70 mg) were put into a small test tube reactor, and 1.0 mL of redistilled dichloromethane was added at room temperature. N-Methylisatin diazo 1a (0.30mmol) was dissolved in 0.7mL redistilled dichloromethane, and injected into the reaction system through a peristaltic pump for 1 hour. After the injection was completed, DBU (0.04mmol) was added, and the reaction was continued for 2h , the reaction was completed, and the solvent was removed by rotary evaporation at 40 ° C, and then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1: 50 ~ 1: 20) to obtain 3,3-spiro (2-tetrahydrofuran) oxidation Indole polycyclic compound 5a. Yield 80%, d.r. 91:9. See Table 1.
[0028]
[0029] The characterization of the present embodiment product 3,3-spi...
Embodiment 2-16
[0032] Embodiment 2-16 prepares compound (5b~5p)
[0033] Embodiment 2-16 is the same as embodiment 1. See Table 1 for the changes of substituents, compound number, d.r. value, yield, etc. in the reaction.
[0034] Table 1
[0035]
[0036] The characterization of the product 3,3-spiro(2-tetrahydrofuran)oxindole polycyclic compound 5b~5p is as follows:
[0037] 5b:
[0038] 1 H NMR (400MHz, CDCl 3 , 25°C, TMS): δ=8.21(d, J=8.2Hz, 1H), 8.03(d, J=7.5Hz, 1H), 7.47(dd, J=11.9, 4.9Hz, 1H), 7.42-7.33 (m, 1H), 7.24(d, J=8.5Hz, 1H), 7.18(s, 1H), 7.12(dd, J=8.7, 2.4Hz, 1H), 6.88(d, J=8.7Hz, 1H) , 6.49(d, J=2.2Hz, 1H), 6.27(s, 1H), 5.30(dd, J=9.1, 2.8Hz, 1H), 4.89(s, 1H), 3.58(s, 3H), 2.77- 2.41(m, 2H), 2.12(s, 3H);
[0039] 13 C NMR (400MHz, CDCl 3 ,25℃,TMS):δ=175.39,169.56,169.49,150.04,140.25,132.27,132.05,131.94,130.05,128.62,127.89,126.71,126.09,126.06,125.21,124.49,122.18,120.45,117.14,99.15,86.29 , 85.55, 72.23, 52.31, 51.58, 34.29, 29.70, 25.69.
[0040] 5c:
[0...
Embodiment 17
[0080] Example 17 Inhibition of Aurora Kinase Activity by 3,3-spiro(2-tetrahydrofuran)oxindole polycyclic compounds 5a-5p of the present invention
[0081] Aurora kinase is necessary for the mitotic process. AURKA plays an important role in the formation of mitotic spindle and centrosome maturation. AURKB is necessary for chromosome segregation and cytoplasmic movement. Studies have shown that inhibiting the activity of Aurora kinase disrupts the cell cycle , prevent cell proliferation, cause apoptosis of many types of tumor cells, and have no effect on non-dividing cells. Finding specific inhibitors of Aurora kinase provides a new method for tumor treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com