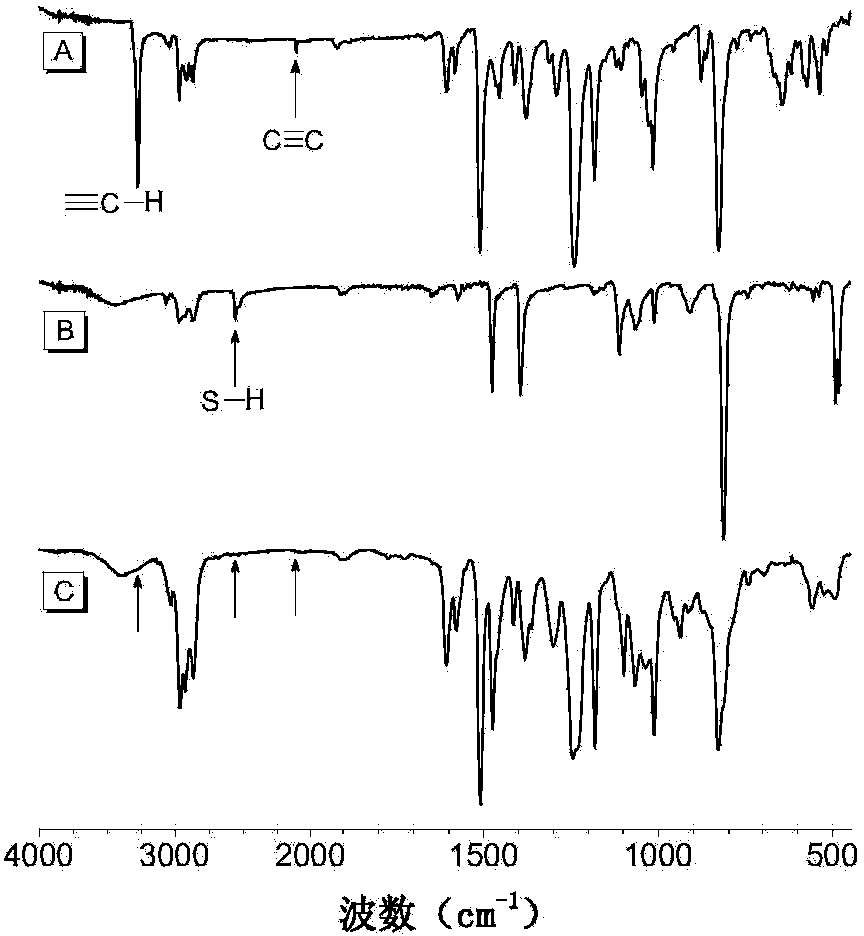
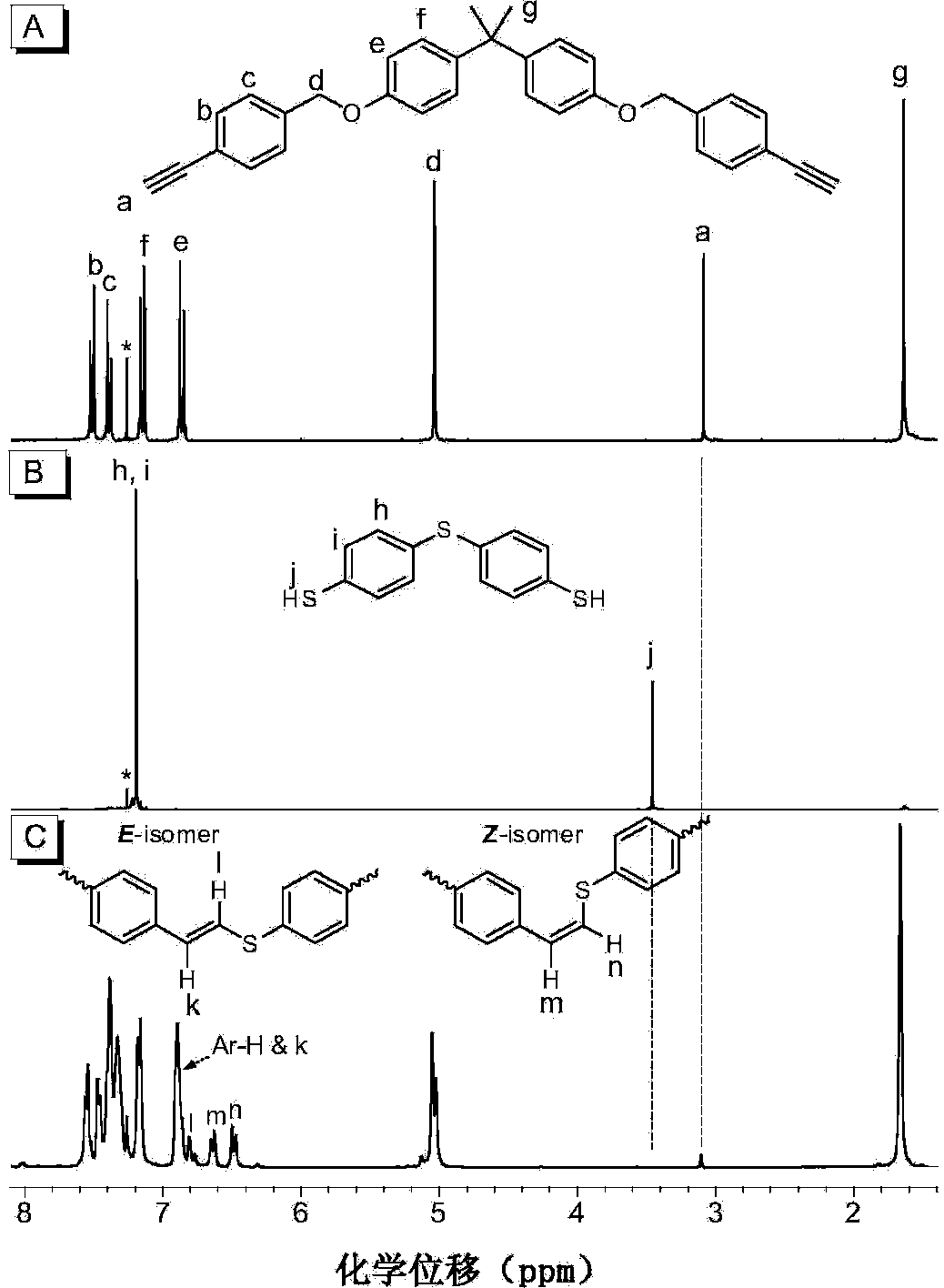
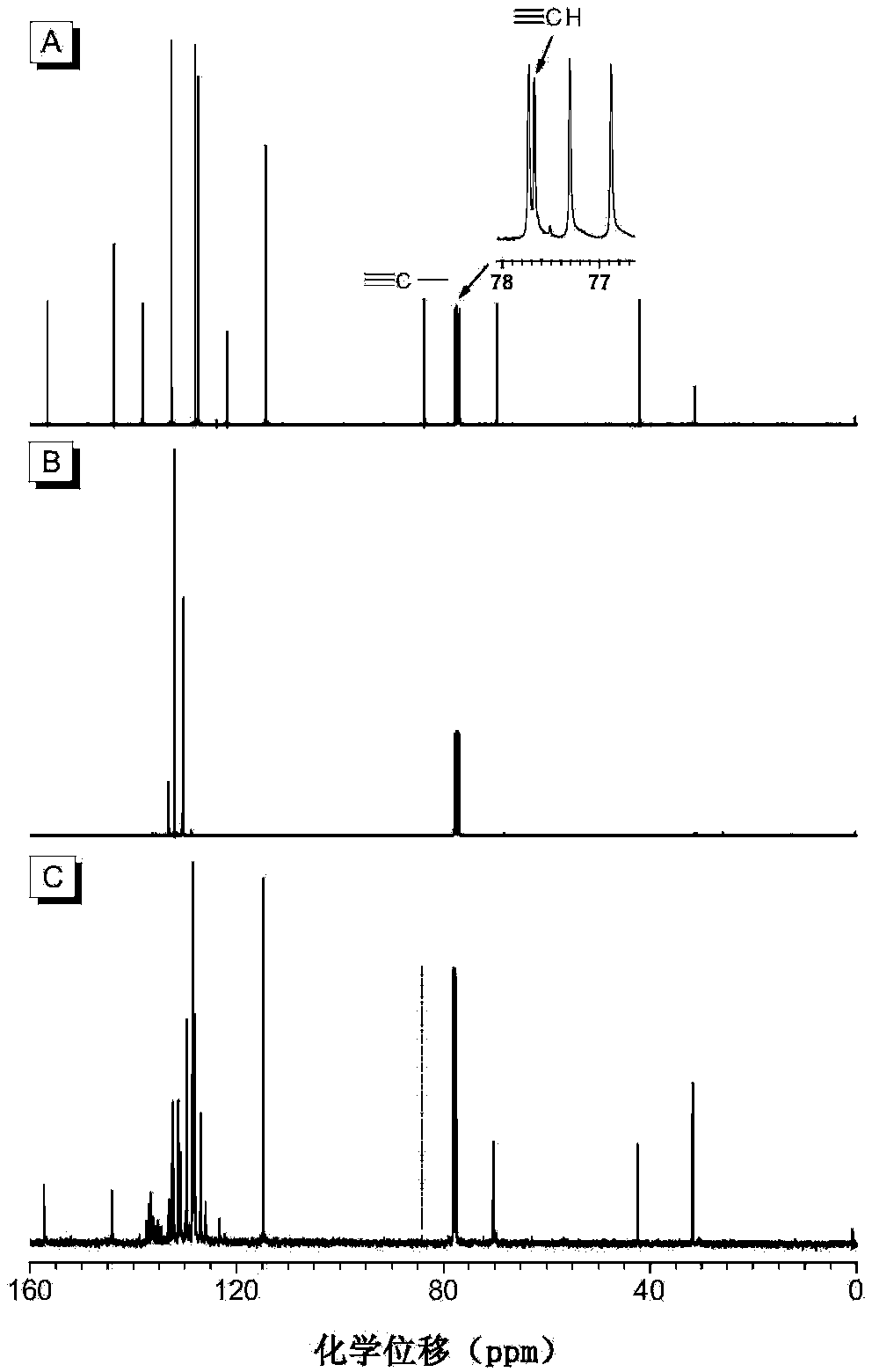
Polyvinylene sulfide and preparation method thereof
A polyvinylene and sulfide technology, which is applied in the fields of polymer chemistry and material science, can solve the problems of increasing the cost of synthetic routes and limiting application fields, and achieves the effects of excellent space selection, simple process, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Synthesis of the first monomer binary alkynyl compound 1a
[0059] Add 1.694g (7.4mmol) of bisphenol A, 4.081g (16.3mmol) of 1-bromo-4-benzylbromobenzene and 2.557g (18.5mmol) of potassium carbonate in a 250mL single-necked flask, add 60mL of acetone, and heat to reflux for 7 hours. After cooling, filtering, and spin-drying, the obtained crude product was purified by thin-layer chromatography and dried in vacuo to obtain 4.128 g of white solid (98.5% yield), which was the first intermediate. 1 H NMR (400MHz, CDCl 3 ),δ(TMS,ppm):7.50(d,4H,Ar-H),7.30(d,4H,Ar-H),7.14(d,4H,Ar-H),6.85(d,4H,Ar-H) H),4.99(s,4H,CH 2 ),1.64(s,6H,CH 3 CCH 3 ).
[0060] Take the first intermediate 3.964g (7mmol), PdCl in the glove box 2 (PPh 3 ) 2 197mg (0.28mmol), CuI107mg (0.56mmol), PPh 3 Add 220mg (0.84mmol) into a 250mL single-necked flask, add 100mL of mixed solvent tetrahydrofuran / triethylamine / piperidine (volume ratio: 60:30:10), after all dissolve, add 3.0mL (21mmol) of trime...
Embodiment 2~5
[0071] Examples 2-5 investigated the influence of different temperatures on reaction conditions. The preparation of polymerized monomers was the same as in Example 1. The reaction conditions and results of step (3) are shown in Table 1.
[0072] Table 1 Effect of Temperature on the Polymerization of Monomers 1a and 2a a
[0073]
[0074] a Reaction in THF under nitrogen atmosphere for 4 hours; [M 0 ]=50mM.
[0075] b T = reaction temperature.
[0076] c Solubility (S) tested in common organic solvents such as THF, chloroform and DMF: √=completely soluble; Δ=partially soluble.
[0077] d Determined by GPC with linear polystyrene as calibrator and THF as mobile phase.
Embodiment 6~9
[0079] Examples 6-9 investigated the influence of different solvents on the reaction conditions. The preparation of polymerized monomers was the same as in Example 1. The reaction conditions and results of step (3) are shown in Table 2.
[0080] Table 2 The influence of solvent on the polymerization of monomer 1a and 2a a
[0081]
[0082] a Reaction under nitrogen atmosphere; T=30℃; [M 0 ]=50mM.
[0083] b t = reaction time.
[0084] c Solubility (S) tested in common organic solvents such as THF, chloroform and DMF: √=completely soluble; Δ=partially soluble.
[0085] d Determined by GPC with linear polystyrene as calibrator and THF as mobile phase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com