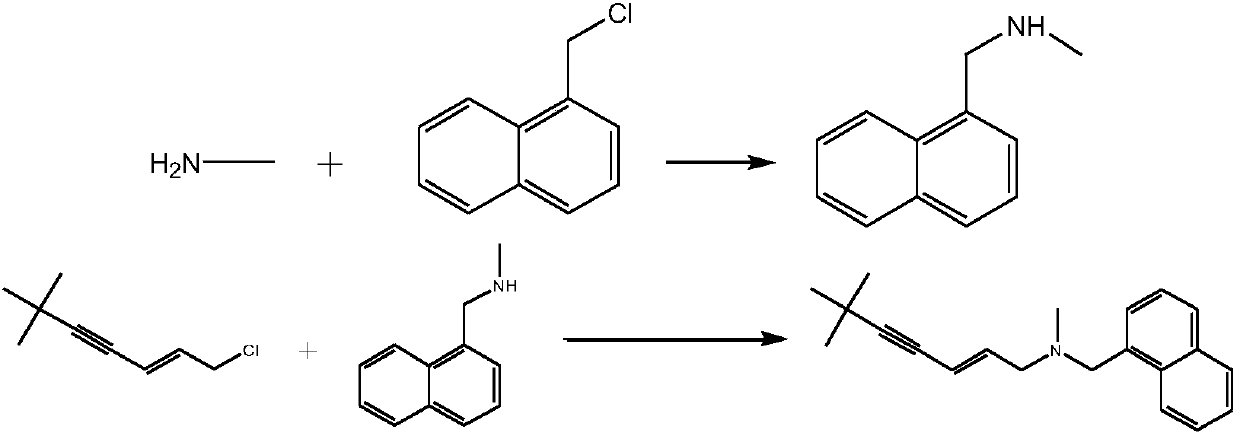
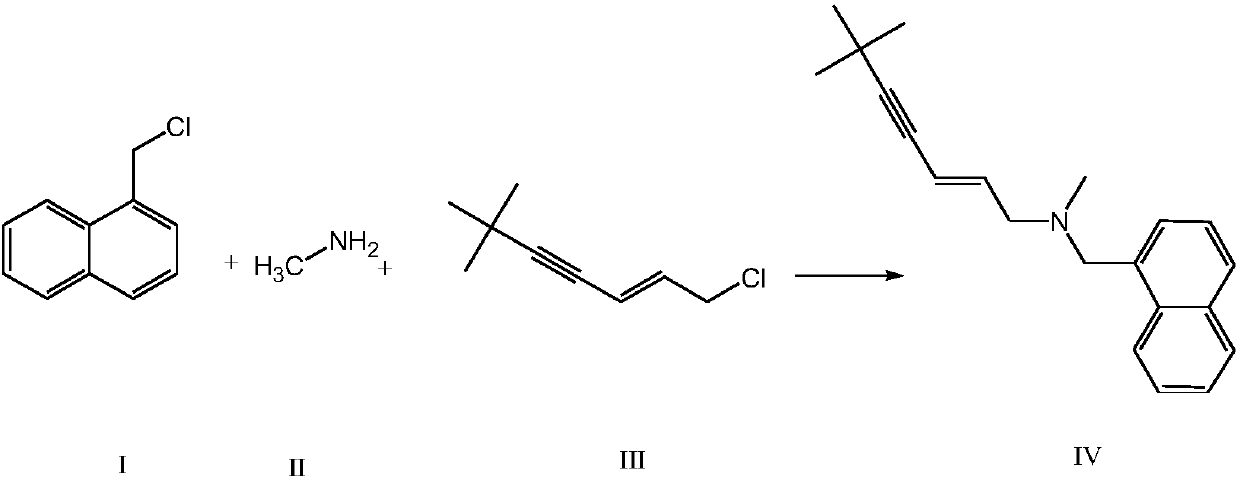
Synthetic method of terbinafine
A technology of terbinafine and a synthesis method, applied in the field of medicinal chemistry synthesis, can solve the problems of many side reactions, low reaction yield and the like, and achieve the effects of simple steps, high yield, and reduction of by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Add 1000ml of purified water to a 2000ml four-necked reaction flask, slowly add 36g of monomethylamine to the water, then add 312g of potassium carbonate to the reaction flask, and add 1-chloromethylnaphthalene to two constant pressure funnels respectively 100g, 93.2g of 1-chloro-6,6-dimethyl-2-hepten-4-yne, temperature controlled at 10°C, slowly drop 1-chloromethylnaphthalene and 1-chloro-6,6-di Methyl-2-heptene-4-yne, control the rate of addition, so that 1-chloromethylnaphthalene and 1-chloro-6,6-dimethyl-2-heptene-4-yne are all added dropwise at the same time, Then, the temperature was controlled at 10°C and the reaction was incubated for 2 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C to concentrate the organic layer under reduced pressure to dryness, add 450ml of ethyl acetate to the residue, heat and reflux for 30 minutes, cool to below 10°C, and suction filte...
Embodiment 2
[0028] Example 2. Add 1000ml of purified water to a 2000ml four-necked reaction flask, slowly add 43.5g of monomethylamine to the water, then add 370.6g of potassium carbonate to the reaction flask, and add 1-chloroform to the two constant pressure funnels respectively Base naphthalene 117.7g, 1-chloro-6,6-dimethyl-2-hepten-4-yne 109.7g, temperature control 20 ℃, slowly drop 1-chloromethylnaphthalene and 1-chloro-6, 6-Dimethyl-2-heptene-4-yne, control the rate of addition, so that 1-chloromethylnaphthalene and 1-chloro-6,6-dimethyl-2-heptene-4-yne are dropped simultaneously After the addition was complete, the temperature was controlled at 20°C and the reaction was kept for 3 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C to concentrate the organic layer under reduced pressure to dryness, add 450ml of ethyl acetate to the residue, heat and reflux for 30 minutes, cool to below 10°C, and...
Embodiment 3
[0029] Example 3. Add 1000ml of purified water to a 2000ml four-necked reaction flask, slowly add 40.4g of monomethylamine to the water, then add 344g of potassium carbonate to the reaction flask, and add 1-chloromethyl to the two constant pressure funnels respectively Naphthalene 109g, 1-chloro-6,6-dimethyl-2-heptene-4-yne 101.8g, temperature control 15°C, slowly drop 1-chloromethylnaphthalene and 1-chloro-6,6- Dimethyl-2-hepten-4-yne, control the rate of addition, so that both 1-chloromethylnaphthalene and 1-chloro-6,6-dimethyl-2-hepten-4-yne are added dropwise at the same time , and then keep the temperature at 15°C for 2.5 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C to concentrate the organic layer under reduced pressure to dryness, add 450ml of ethyl acetate to the residue, heat and reflux for 30 minutes, cool to below 10°C, and suction filter to obtain The white solid was dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com