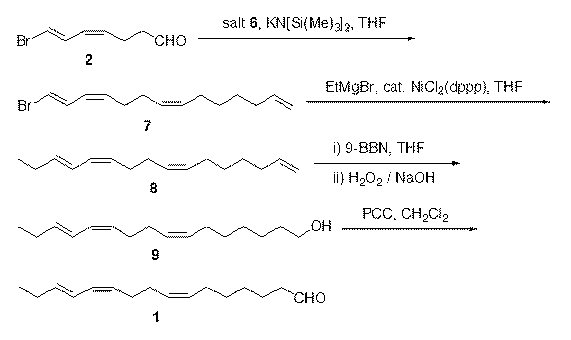
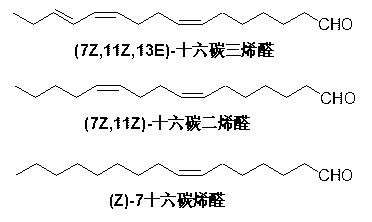
Synthesis method of (7Z,11Z,13E)-hexadecatrienal
A technology of hexadecatrienal and hexadecatriene, which is applied in the field of chemical synthesis of hexadecatrienal, the main component of sex pheromone of citrus leafminer moth, to reduce synthesis cost, high stereoselectivity, Effect of High Isomeric Purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
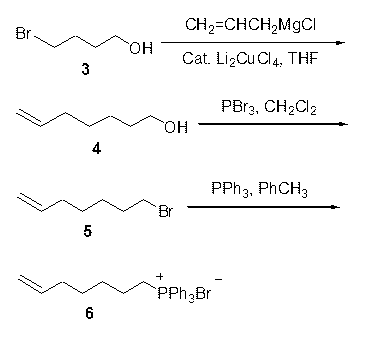
[0020] Example 1 6-hepten-1-ol ( 4 ) Preparation: 4-bromo-1-butanol (15.3 g, 0.1 mol) was dissolved in 100 ml of anhydrous tetrahydrofuran, and allyl magnesium chloride (0.25 mol, 250ml, 1 M) of anhydrous tetrahydrofuran was added dropwise at 0℃ Solution and lithium tetrachlorocuprate (10 mmol, 0.2 mol / L, 50 ml) in anhydrous tetrahydrofuran solution, continue the reaction for 15 hours, add an appropriate amount of saturated ammonium chloride solution to stop the reaction, distill off the tetrahydrofuran, add ethyl acetate for extraction Three times, the organic layer was dried over anhydrous sodium sulfate and the solvent was evaporated to obtain a crude product, which was distilled under reduced pressure to obtain 6-hepten-1-ol ( 4 ) 9.8 g, yield 86%.
Embodiment 2
[0021] Example 2 7-bromo-1-heptene ( 5 Preparation of ): 6-hepten-1-ol (9.1 g, 80 mmol) was dissolved in 80 ml of dry dichloromethane, phosphorus tribromide (8.13 g, 30 mmol) was added dropwise, and after dropping, continue under ice bath After stirring for 3 hours, add saturated sodium carbonate to neutralize to pH=7-8, separate the organic layer, extract the aqueous layer with dichloromethane once, combine the organic layers, dry over anhydrous sodium sulfate and evaporate the solvent under reduced pressure The crude product was obtained, which was distilled under reduced pressure to obtain 7-bromo-1-heptene ( 5 ) 11.9 g, yield 84%.
Embodiment 3
[0022] Example 3 (6-heptenyl)triphenylphosphonium bromide ( 6 ) Preparation: 7-bromo-1-heptene (11.5 g, 65 mmol) and triphenylphosphine (20.4 g, 78 mmol) were dissolved in 130 ml of toluene, reacted under reflux for 48 hours, and a solid precipitated out, filtered and washed with toluene Twice, the solid was collected and dried under vacuum to obtain 27.1 g of (6-heptenyl)triphenylphosphonium bromide with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com