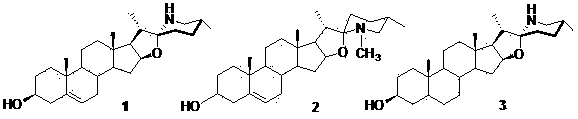
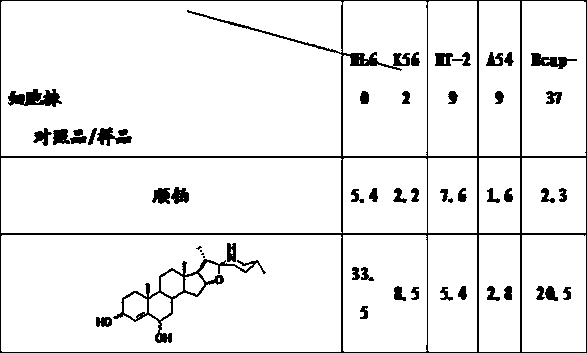
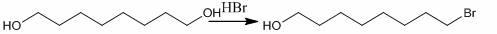Patents
Literature
38 results about "Pyridinium chlorochromate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
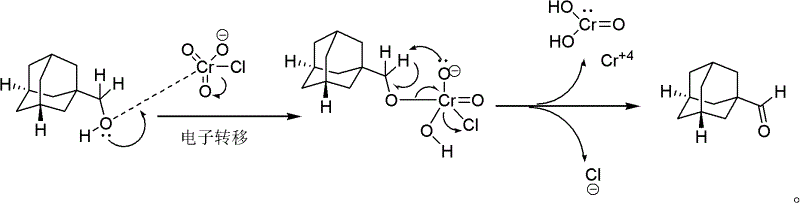
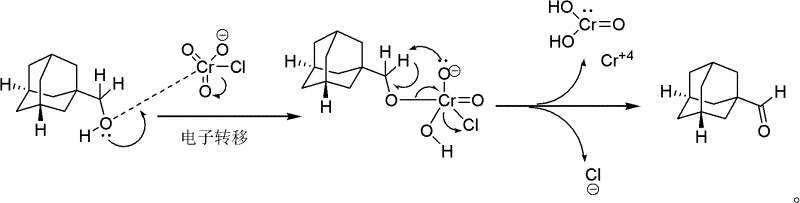
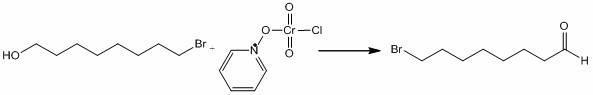
Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C₅H₅NH]⁺[CrO₃Cl]⁻. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
Method and test strip of detecting oxidizing adulterant in urine
A single reagent system and a method to detect and measure oxidizing adulterants in bodily fluid being screened for drugs of abuse are disclosed. The system comprising a strip containing 0.05 to 0.2 micromole / 25 sq. mm. of a benzidine derivative and is used to detect sodium hypochlorite (bleach), chlorine, hydrogen peroxide, sodium bromide, sodium iodide, sodium nitrite, and pyridinium chlorochromate adulterants in urine, sweat, saliva, blood or other bodily fluids during screening for drugs of abuse.
Owner:BRANAN MEDICAL
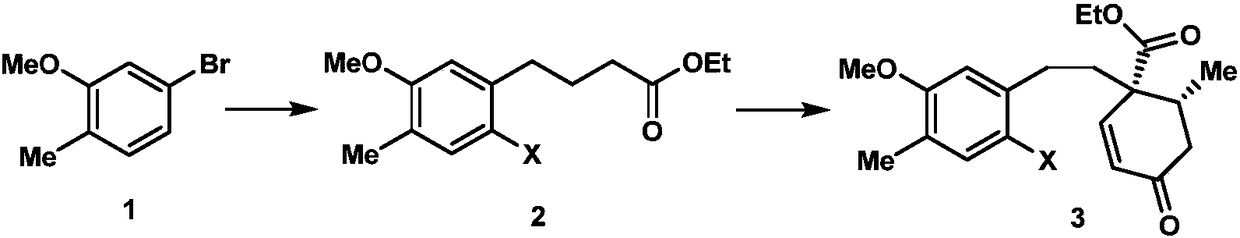
Synthetic method of diamondback moth sex pheromone compound
InactiveCN102795997ALow priceReduce manufacturing costOxygen-containing compound preparationOrganic compound preparationHydrolysisToxicology
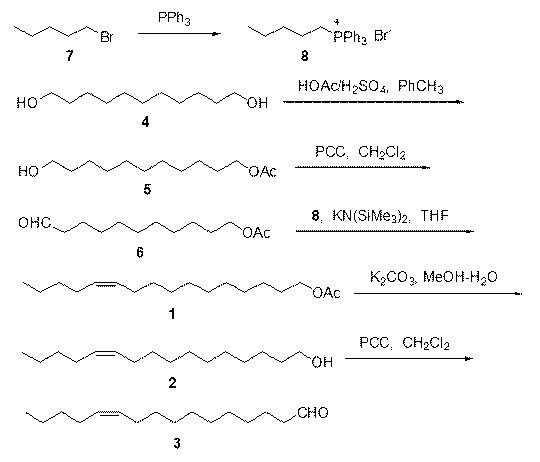
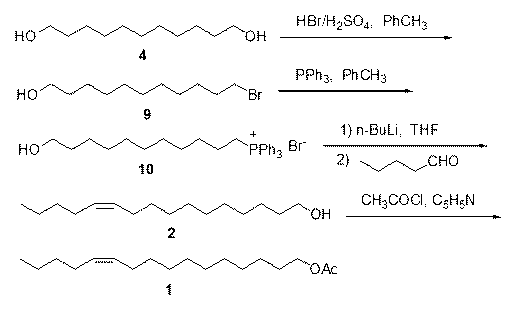
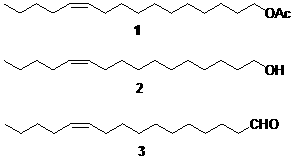
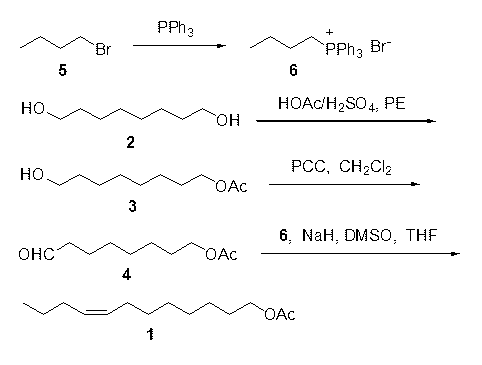
The invention discloses two synthetic methods of a diamondback moth sex pheromone compound. The first method adopts 1,11-undecadiol and n-amyl bromide as initial raw materials, and comprises four-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-yl acetate, a hydrolytic reaction to obtain (Z)-11-hexadecen-1-ol, and a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal. The second method adopts 1,11-undecadiol and valeraldehyde as initial raw materials, and comprises three-step reactions including a critical Wittig reaction to synthesize (Z)-11-hexadecen-1-ol, a pyridinium chlorochromate oxidation reaction to obtain (Z)-11-hexadecenal, and an esterification reaction to obtain (Z)-11-hexadecen-1-yl acetate. The invention has the advantages of cheap and easily available raw materials, simple synthetic route, mild reaction condition, convenient and safe operation, high yield, and low cost.
Owner:昆明博鸿科技有限公司
Method for synthesis of sex pheromone compound of grapholitha molesta
InactiveCN102795998ALow priceRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid esters preparationOrganic baseBiology
The invention discloses a method for synthesis of a sex pheromone compound of grapholitha molesta. The method is characterized in that 1,8-octanediol and n-butylbromide are used as starting materials; 1,8-octanediol undergoes a monoesterification reaction to produce 8-hydroxyoctyl acetate; 8-hydroxyoctyl acetate is oxidized into 8-oxo-octyl acetate by pyridinium chlorochromate; and n-butyltriphenylphosphonium bromide produced by a reaction of n-butylbromide and triphenylphosphine is treated by organic bases and then undergoes a Wittig reaction with 8-oxo-octyl acetate to produce (Z / E)-8-dodecenyl acetate. The method provided by the invention has the advantages that raw materials are cheap and easily available; a synthesis route is simple; reaction conditions are mild; operation is convenient and safe; a yield is high; and a cost is low.
Owner:昆明博鸿科技有限公司
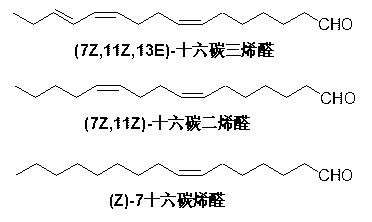
Synthesis method of (7Z,11Z,13E)-hexadecatrienal
InactiveCN102795977ALow costLower synthesis costCarbonyl compound preparation by oxidationPhenyl groupToxicology
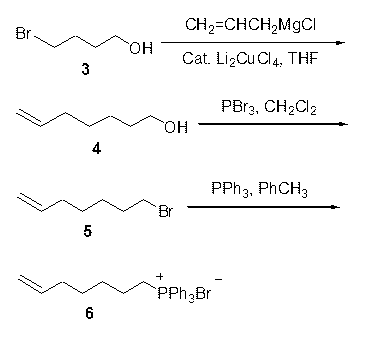
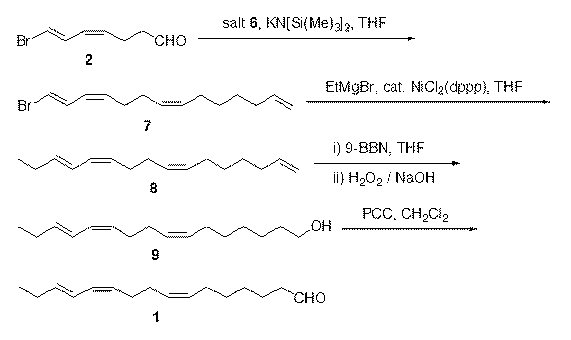
The present invention relates to a synthesis method of (7Z,11Z,13E)-hexadecatrienal as main constituent of citrus leaf-miner sex pheromone. 7-Bromo-(4Z,6E)-heptadienal and 4-bromo-1-butenol are used as start raw materials, the 4-bromo-1-butenol is coupled with allylmagnesium bromide to obtain 6-hepten-1-ol and then the 6-hepten-1-ol is brominated and salified with triphenylphosphine to obtain 1-bromo-(1E,3Z,7Z,13)-tetradecatetraene, and then the 1-bromo-(1E,3Z,7Z,13)-tetradecatetraen is subjected to Kumada coupling to obtain (1,7Z,11Z,13E)-hexadecatetraene, and then the (1,7Z,11Z,13E)-hexadecatetraene is subjected to hydroboration-oxidation reaction to obtain (7Z,11Z,13E)-hexadecatrienol, and finally the (7Z,11Z,13E)-hexadecatrienol is oxidized into (7Z,11Z,13E)-hexadecatrienal by pyridinium chlorochromate. The synthesis method of (7Z,11Z,13E)-hexadecatrienal has advantages of cheap and easily obtained raw materials, simple synthesis path, mild reaction conditions, high yield, low cost, convenient and safe operation.
Owner:昆明博鸿科技有限公司
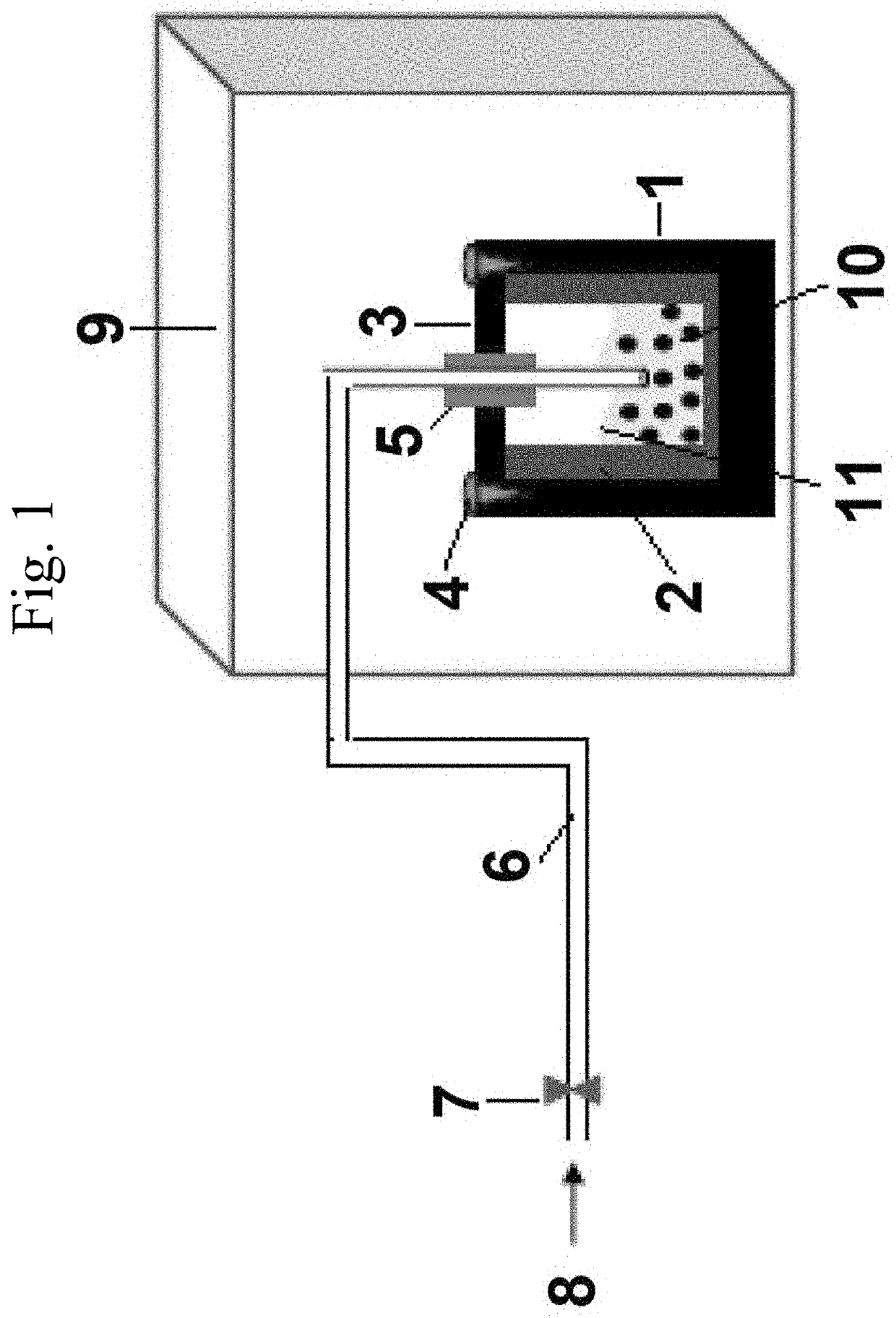
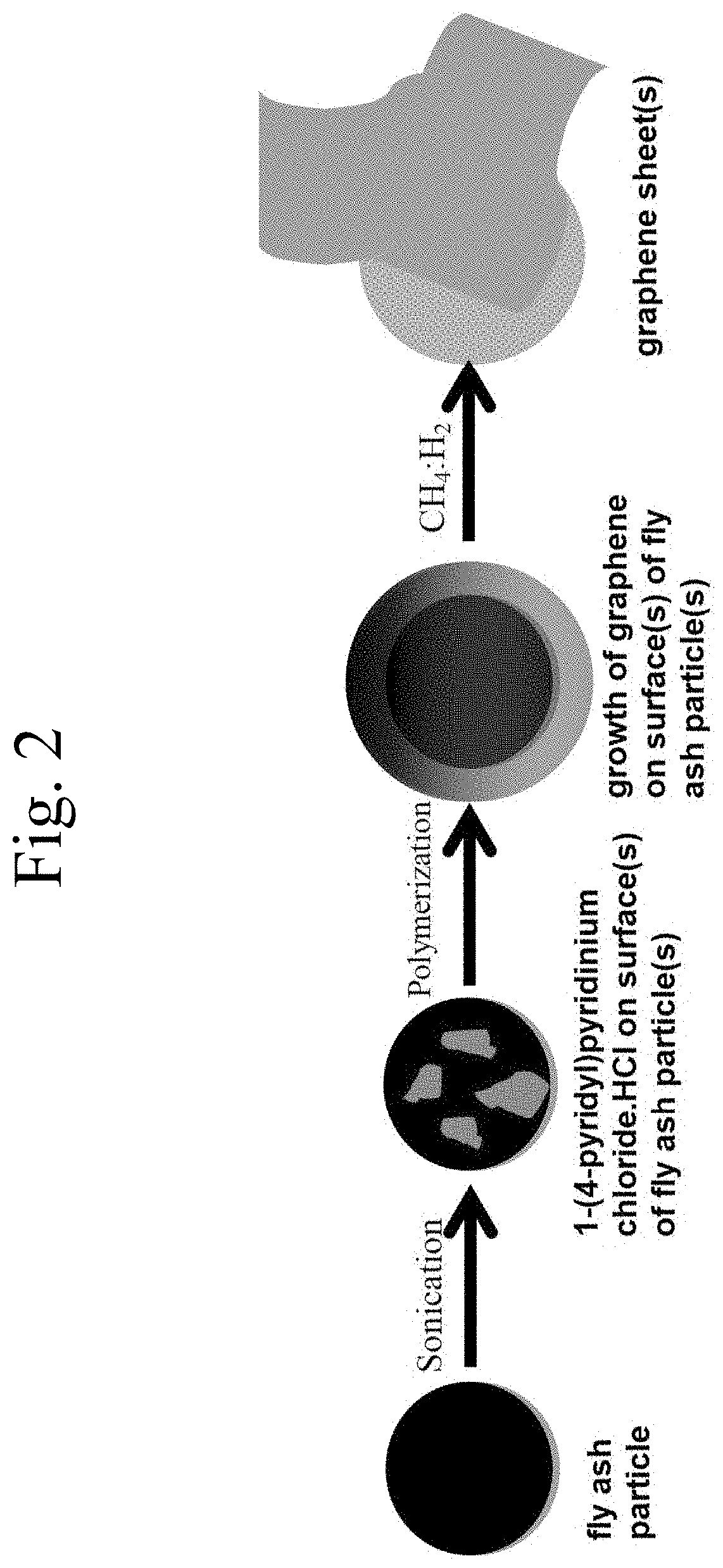
Process for forming graphene-coated particles
Methods of forming graphene may include reacting a dispersed mixture, comprising fly ash, a charged heteroaromatic compound, particularly a pyridinium compound, such as a 1-(4-pyridyl)-pyridinium salt, and a solvent, particularly an alcohol, such as ethanol, with a polymeric oxidizing agent, preferably polymer-supported pyridinium chlorochromate, to form a second mixture; and contacting the second mixture at a temperature of 120 to 180° C. with a gas stream comprising at least 0.1 vol. % CH4 and at least 10 vol. % H2 to form graphene on the fly ash. Methods of managing waste may comprise using fly ash waste to produce graphene. Devices for implementing such methods may involve steel cylindrical reaction vessels including a cover through which a valve-stoppable pipe is fed, which reaction vessel is at least partially surrounded by a heating device, and suitable for handling solvent and fly ash, as well as for receiving gas inflow through the pipe.
Owner:KING ABDULAZIZ UNIV
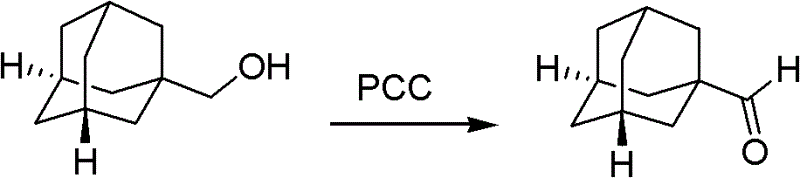
Adamantane formaldehyde synthetic method suitable for industrial production
InactiveCN102617310AMild reaction conditionsEasy to operateCarbonyl compound preparation by oxidationSorbentSilica gel
An adamantane formaldehyde synthetic method suitable for industrial production includes that an adamantane methanol is used as a raw material, a dichloromethane is used as a solvent, a pyridinium chlorochromate (PCC) is used as an oxidizing agent, simultaneously diatomites or silica gels are added to be used as adsorbing agents, a reaction is performed at the temperature of 5 DEG C to 40 DEG C for one to three hours, then post-processing is performed by adding calcium hydroxides and the silica gels or the diatomites in a reaction liquid, and the adamantane formaldehyde is obtained. By means of the adamantane formaldehyde synthetic method suitable for industrial production, the PCC is used for oxidizing the adamantane methanol to obtain the adamantane formaldehyde, and the reaction is performed at room temperature so that reaction conditions are mild. Besides, the used solvent is easy to obtain, the operation process is simple, the separation is convenient, and the yield is high, thereby the adamantane formaldehyde synthetic method is successfully applied to industrial production of onglyza at present.
Owner:TIANJIN WEIJIE TECH
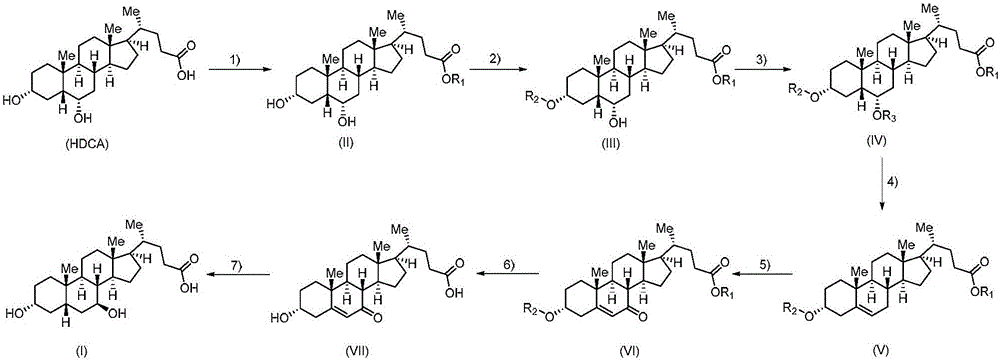
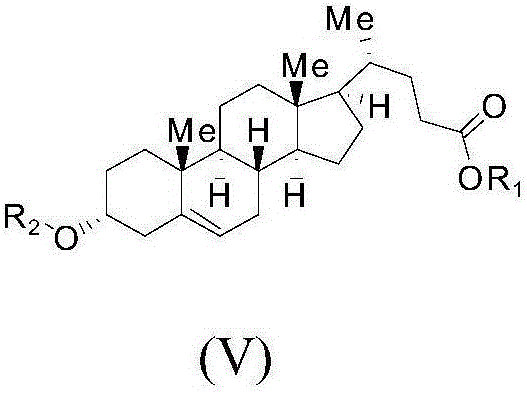
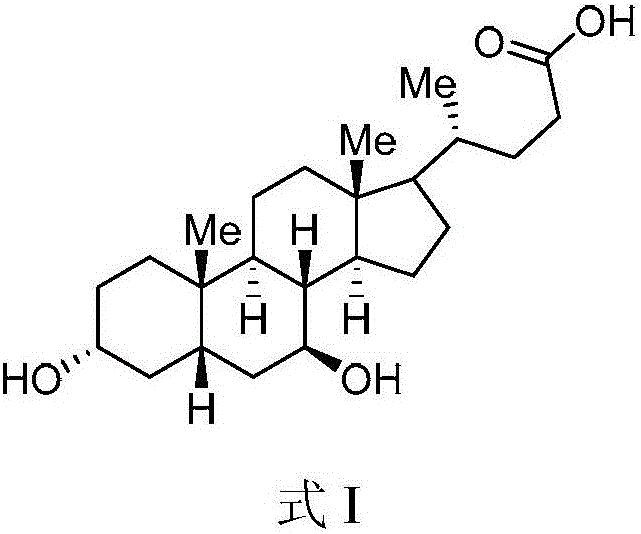
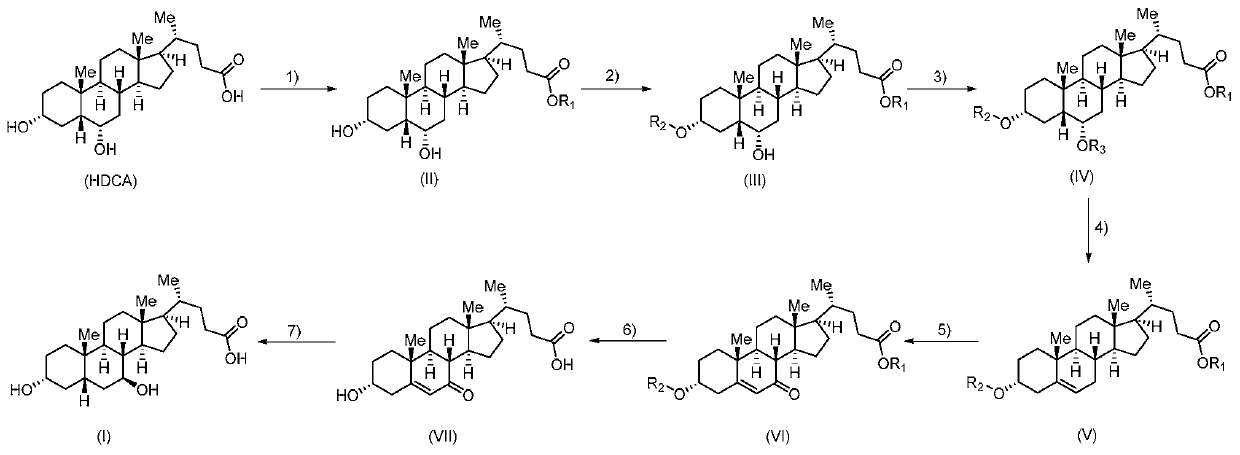
Method for preparing ursodeoxycholic acid
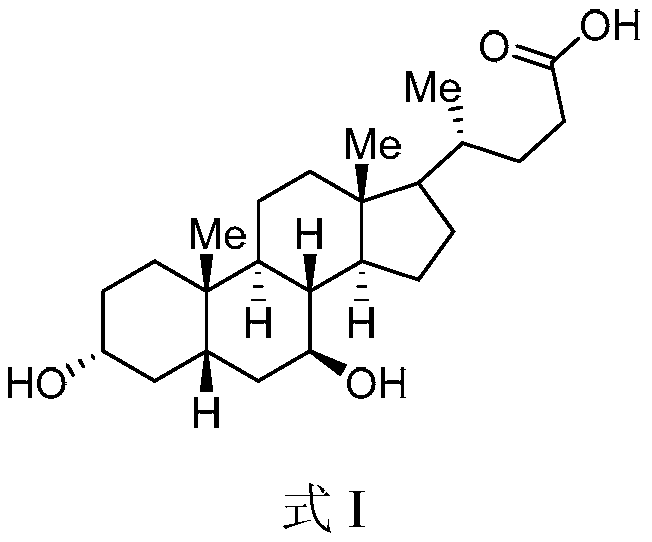
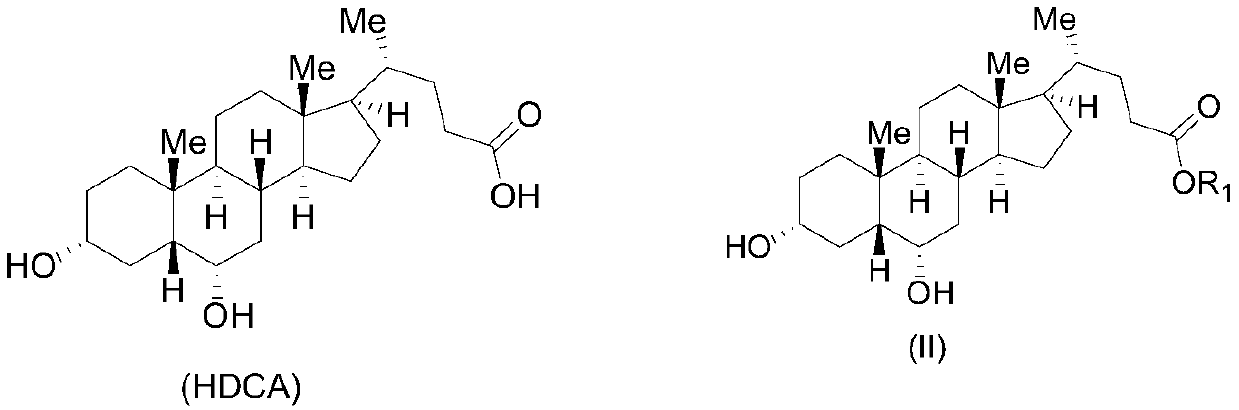
The invention belongs to the field of medicine, and particularly relates to a method for preparing an ursodeoxycholic acid. The method comprises the following steps: performing esterification reaction to obtain a compound shown in formula II by taking an HDCA (hyodeoxycholic acid) as a starting raw material; performing selective hydroxy protection reaction to obtain a compound shown in formula III; performing hydroxy protection reaction to obtain a compound shown in formula IV; performing elimination reaction to obtain a compound shown in formula V; performing oxidation reaction on the compound shown in formula V under the action of tert-butyl hydroperoxide and pyridinium chlorochromate to obtain a compound shown in formula VI; performing hydrolysis to obtain a compound shown in formula VII; performing reduction to obtain the ursodeoxycholic acid shown in formula I. According to the method, the ursodeoxycholic acid is prepared by taking the HDCA as the starting raw material, so that not only can the problem of raw material shortage be solved, but also the method is convenient to operate, low in byproduct rate and cost, mild in reaction condition and suitable for large-scale production of the ursodeoxycholic acid.
Owner:青州市欣泰生物制品有限公司
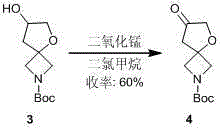
2-tertbutyloxycarbonyl-7-carbonyl-5-O-2-azaspiro(3.4)octane synthesis method
InactiveCN105017268AReasonable reaction process designShort synthetic routeOrganic chemistryTert-Butyloxycarbonyl protecting groupSodium hydrogen sulphite
The present invention relates to a 2-tertbutyloxycarbonyl-7-carbonyl-5-O-2-spiro(3.4)octane synthesis method. A purpose of the present invention is mainly to solve the technical problems of high price of the reagent used in the existing synthesis route, high risk and no suitability for industrial production. According to the present invention, the three-step method is used to synthesize, wherein the first step is that a saturated ammonium chloride aqueous solution of zinc powder is added to a tetrahydrofuran solution of 1-tertbutyloxycarbonyl-3-azetidinone, then allyl bromide is added in a dropwise manner, and the reaction solution reacts at a room temperature overnight to obtain 1-tertbutyloxycarbonyl-3-allyl-azetidinol, the step is that an aqueous solution of sodium hydrogen sulfite is added to an acetonitrile aqueous solution of the 1-tertbutyloxycarbonyl-3-allyl-azetidinol and sodium periodate, and stirring is performed for 16 h at a temperature of 80 DEG C to obtain 2-tertbutyloxycarbonyl-7-hydroxy-5-O-2-azaspiro(3.4)octane, and the third step is that pyridinium chlorochromate is added to a dichloromethane solution of the 2-tertbutyloxycarbonyl-7-hydroxy-5-O-2-azaspiro(3.4)octane under ice bath, and a reaction is performed at a room temperature overnight to obtain the 2-tertbutyloxycarbonyl-7-carbonyl-5-O-2-spiro(3.4)octane.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
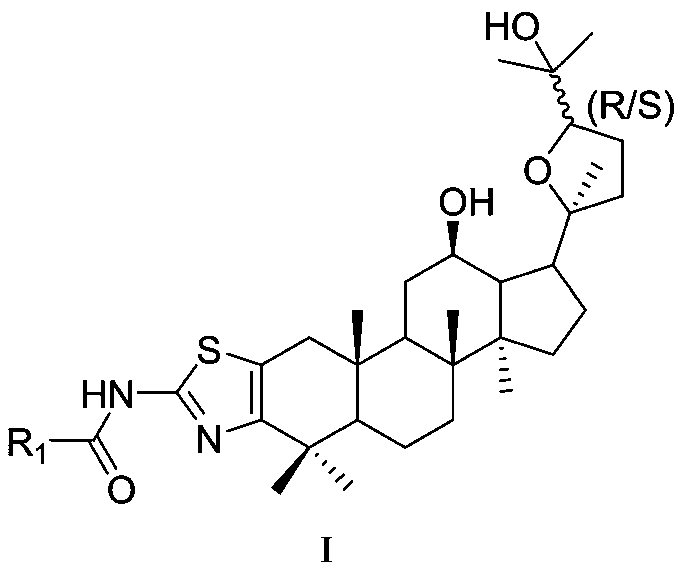
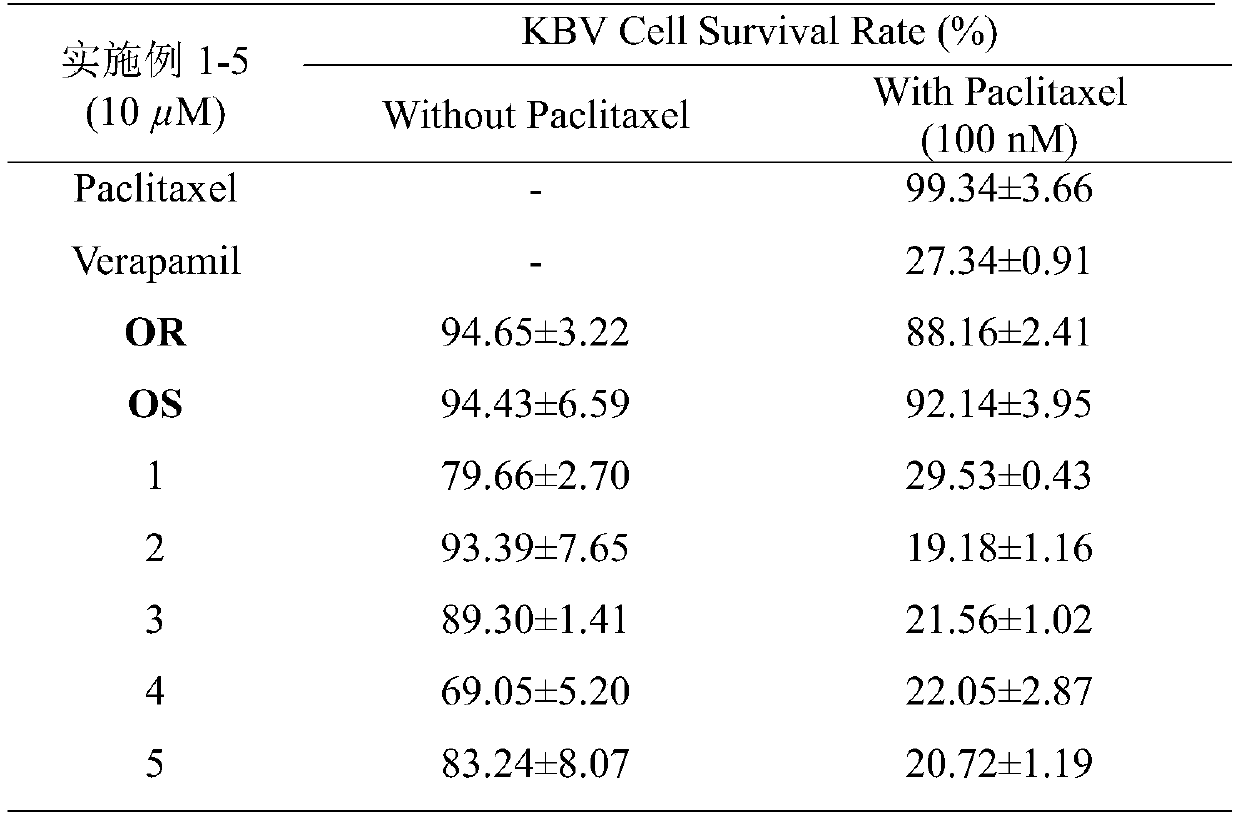
Ocotillol type ginseng sapogenin A ring-aminothiazole ring derivative and preparation method thereof
ActiveCN111471080AIncreased sensitivityGood antitumor activityOrganic active ingredientsSteroidsAcetic anhydrideProtopanaxadiol
The invention discloses an ocotillol type ginseng sapogenin A ring-aminothiazole ring derivative and a preparation method thereof. The product provided by the invention is prepared according to the following method including: a) by taking 20(S)-protopanaxadiol as a raw material, in the presence of DMAP, protecting 3,12-OH by acetic anhydride, oxidizing by m-CPBA, and removing a protecting group byalkaline hydrolysis to obtain (20S,24R)-epoxy dammar-3beta,12beta,25-triol (OR) and (20S,24S)-epoxy dammarane-3beta,12beta,25-triol (OS); b) respectively oxidizing OR and OS by pyridinium chlorochromate hydrochloride, and then sequentially reacting with pyridinium bromide perbromide and thiourea; c) under the alkaline condition, protecting acid having amino at the terminal end with Boc anhydride;d, in the presence of organic alkali and a condensing agent, reacting the product obtained in the step b with Boc-amino acid; and e) removing Boc under an acidic condition. The invention also discloses a preparation method of the derivatives and a novel application of the derivatives in tumor drug resistance reversion.
Owner:YANTAI UNIV
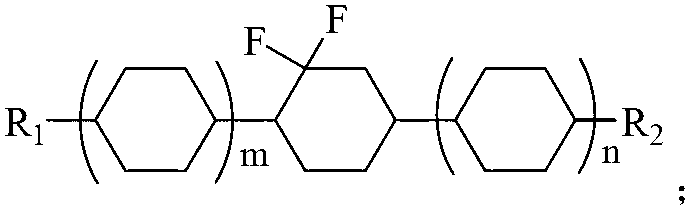
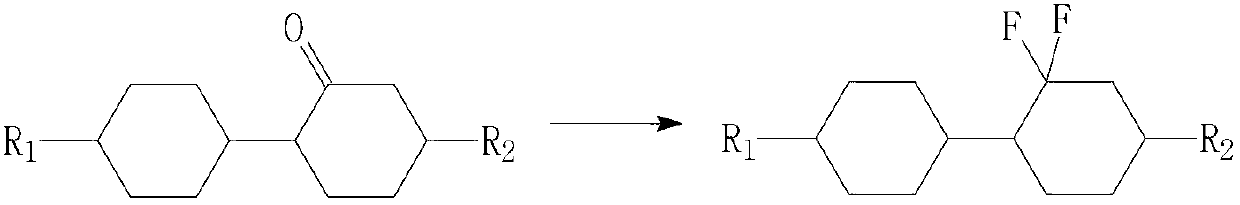
Preparation method of difluorocyclohexane liquid crystal compound
InactiveCN102838458AReduce pollutionEasy to operateOrganic compound preparationHalogenated hydrocarbon preparationAfter treatmentClosed loop
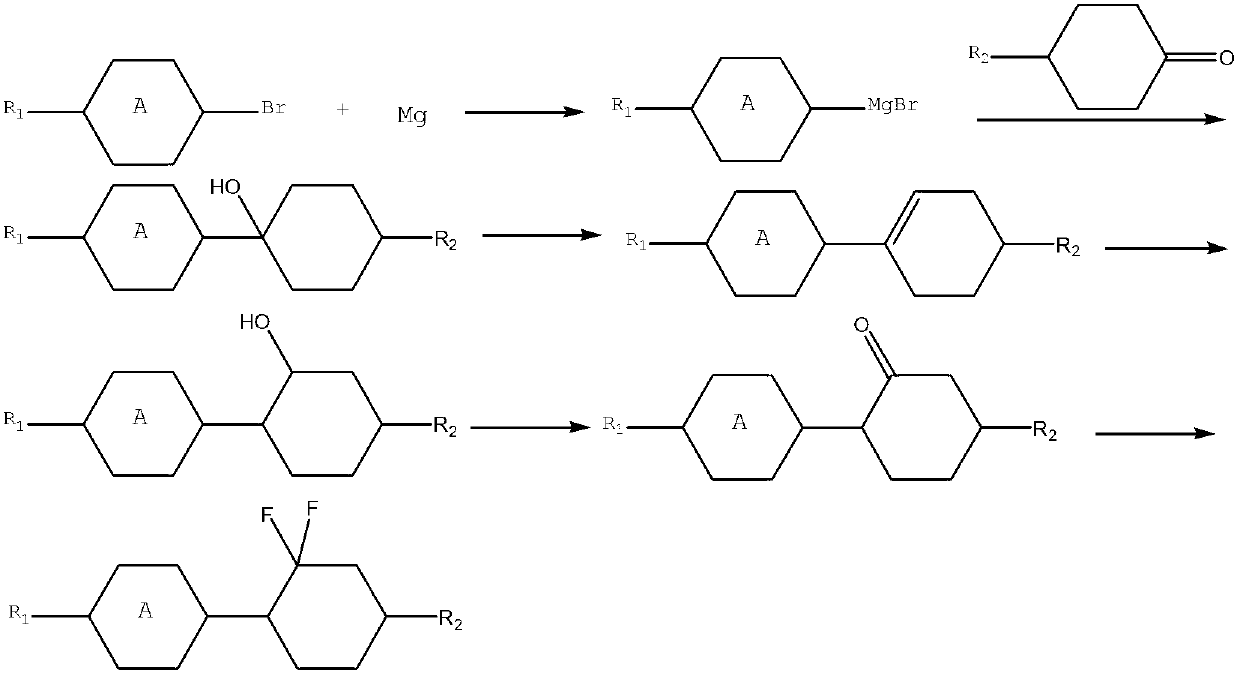
The invention relates to a preparation method of a difluorocyclohexane liquid crystal compound. The preparation method is mainly prepared through closed loop hydrogenation of two substances shown in the specification, wherein R1 and R2 are alkyl groups with carbon atom number of 1-12 or alkoxyl groups with carbon atom number of 1-13; n is 0 or 1, m is 0 or 1, and n and m are not 0 at the same time. The method has the advantages of simple operation in each step, mild reaction conditions, particularly no need of reducer borane and oxidant such as PCC (Pyridinium Chlorochromate) and capabilities of avoiding a large amount of wastewater produced in after-treatment, saving cost and also greatly reducing environmental pollution.
Owner:BEIJING BAYI SPACE LCD MATERIALS TECH
Method for synthesizing sex pheromone of Plutella xylostella
InactiveCN108402041AFew synthetic stepsThe synthesis process is simpleBiocideOrganic compound preparationChemical synthesisAcetic anhydride
The invention belongs to the technical field of chemical synthesis, and particularly relates to a method for synthesizing sex pheromone of Plutella xylostella, and the method comprises the following steps: 1) reacting 11-bromo-1-undecanol with triphenylphosphine in an organic solvent to form bromo-hendecanol quaternary phosphonium salt; 2) under nitrogen protection, reacting the bromo-hendecanol quaternary phosphonium salt obtained in the step 1) with n-pentanal and sodium ethoxide in a tetrahydrofuran solvent to form cis-11-hexadecenol; 3) in a dichloromethane solution, oxidizing the cis-11-hexadecenol obtained in the step 2) with pyridinium chlorochromate to form cis-11-hexadecenal; 4) in dichloromethane, reacting the cis-11-hexadecenol obtained in the step 2) with acetic anhydride and pyridine to form cis-11-hexadecenol acetate. The method uses the cheap and easily-available 11-bromo-1-undecanol as a starting material, reduces the synthesis step, simplifies the synthesis process, has high product yield, mild and controllable reaction conditions and low requirements on equipment, and can be used for industrial production.
Owner:WUHAN CHUQIANG BIOLOGICAL TECH CO LTD
Synthesis of plodia interpunctella sex pheromone 9Z, 12E-tetradecadiene-1-acetate
InactiveCN103626657ALow priceRaw materials are cheap and easy to getOrganic compound preparationGroup 5/15 element organic compoundsOrganic basePhenyl group
The invention discloses a synthesis method of plodia interpunctella sex pheromone 9Z, 12E-tetradecadiene-1-acetate. According to the synthesis method, 1, 9-nonanediol and 3E-pentene-1-ol are taken as initial raw materials. The synthesis method comprises following steps: 9-hydroxy nonanol acetate is obtained via single esterification of 1, 9-nonanediol; a key intermediate 9-oxo nonanol acetate is obtained via oxidation of pyridinium chlorochromate; 5-bromo-3E-pentene is obtained via bromination of 3E-pentene-1-ol; 3E-pentenyl benzyltriphenylphosphonium bromide is obtained via reaction of 5-bromo-3E-pentene with triphenylphosphine; and 3E-pentenyl benzyltriphenylphosphonium bromide is processed with an organic acid, and then is subjected to Wittig reaction with 9-oxo nonanol acetate so as to obtain (9Z, 12E)-tetradecadiene-1-acetate. Advantages of the synthesis method are that: raw materials are cheap and easily available; synthesis route is simple; reaction conditions are mild; operation is convenient and safe; yield is high; and cost is low.
Owner:昆明博鸿科技有限公司
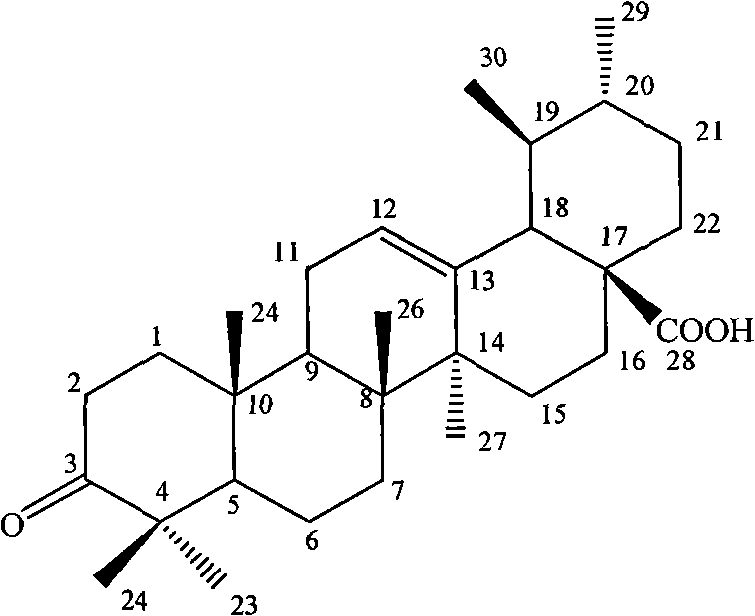
Method for preparing ursolic acid derivative 3-o-keto-12-alkenyl-28-ursolic acid
The invention relates to the field of natural medicaments, in particular to a method for preparing 3-o-keto-12-alkenyl-28-ursolic acid. The method is characterized by performing after-treatment on reaction solution to improve the reaction solution on the basis of the conventional reaction method, namely reacting ursolic acid with pyridinium chlorochromate. The after-treatment method of the invention comprises the following steps of: concentrating the reaction solution under reduced pressure; extracting the residual solid by using ethyl ether or butanol; concentrating an organic phase under reduced pressure to obtain a crude product; and allowing the crude product to pass through a silicagel column for elution to obtain the 3-o-keto-12-alkenyl-28-ursolic acid. The preparation method of the invention has the advantages of simple separation method, easy operation and high product yield.
Owner:ARMY MEDICAL UNIV
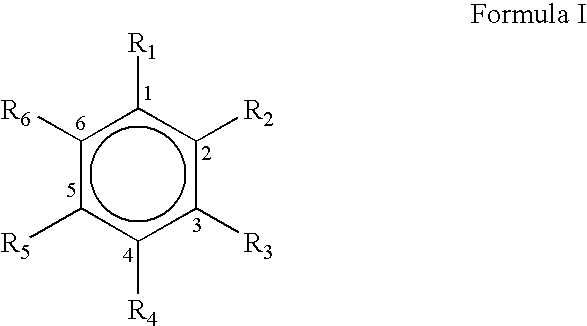
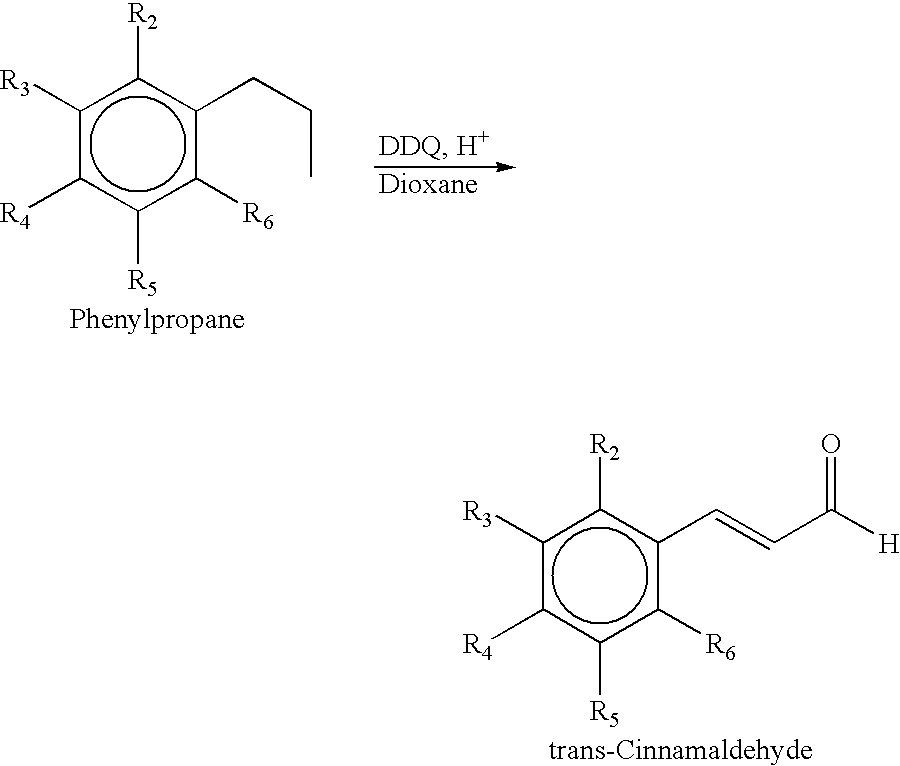
Process for the preparation of substituted trans-cinnamaldehyde, a natural yellow dye, from phenylpropane derivatives
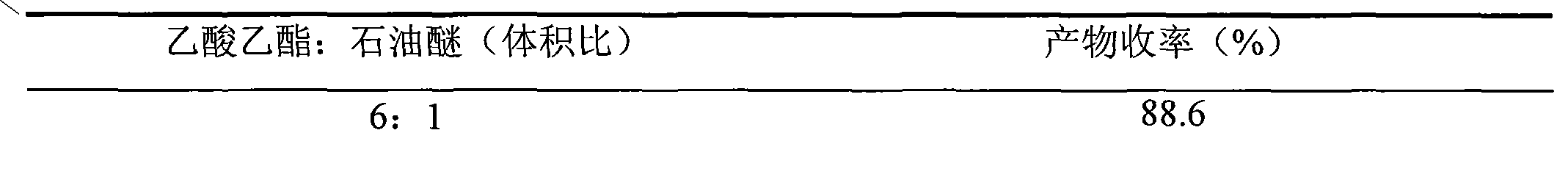
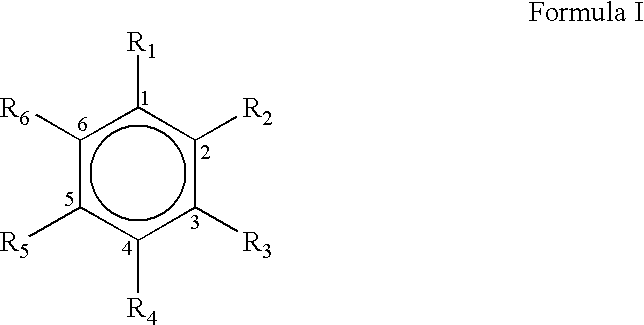
The present invention relates to the preparation of substituted trans-cinnamaldehyde, a natural yellow dye from Phenylpropane derivatives having R2-R3-R4-R5-R6 substitution, wherein R2 to R6 equal or different, being hydrogen or hydroxy or acyl or halogen or alkyl or heterocyclic or aryl or dioxymethylene or alkoxy groups, etc., by oxidizing the said phenylpropane derivatives using a oxidising agent such as 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) or p-chloranil or pyridinium chlorochromate (PCC) or tBuOOH or CrO3 with a catalytic amount of inorganic / organic acid or alumina, celite, and silica gel as a solid support for microwave irradiation and thus substituted trans-cinnamaldehydes, a natural yellow dye, are obtained in high yield ranging from 68-82%.
Owner:COUNCIL OF SCI & IND RES
Environment-friendly wood flame retardant
The invention discloses an environment-friendly wood flame retardant, and belongs to the field of flame-retardant materials. According to the environment-friendly wood flame retardant, fibers with different particle diameters are treated by alkali liquid, part of hemicellulose and lignin in bagasse are destroyed, the degree of crystallinity of the bagasse is decreased, the reactivity of the fibersis improved, C-C of a vicinal diol structure in an oxidable molecular structure of sodium periodate is broken, two aldehyde groups are generated, part of hydroxyl in the cellulose structure is converted into the aldehyde groups through pyridinium chlorochromate under the effect of CH2Cl2, the adhesion force of the flame retardant on the surface of the wood is improved, and the flame retardant effect is effectively maintained for a long period. Ions generated after ionization of metal salt components inside the system in water can be adsorbed to the surface of an interface film, metal salt isgradually separated out while moisture is reduced under the heat action, the ferric oxide has a certain dehydration effect, and the moisture is generated for heat absorption and flame retarding. The environment-friendly wood flame retardant solves the problems that a commonly-used flame retardant can generate toxic smoke in the case of fire at present, and the flame retarding effect is not good.
Owner:FOSHAN CHANCHENG DISTRICT NUOGAO ENVIRONMENTAL PROTECTION TECH CO LTD
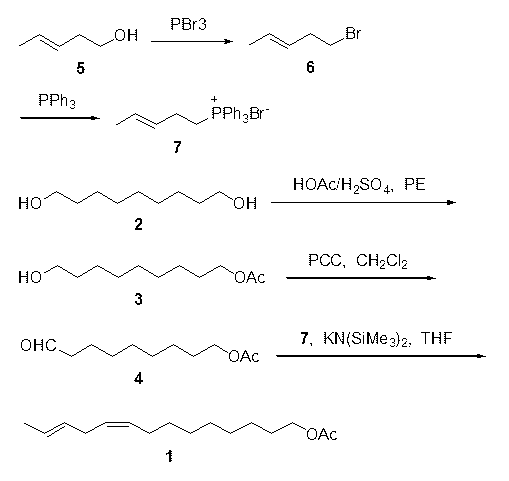
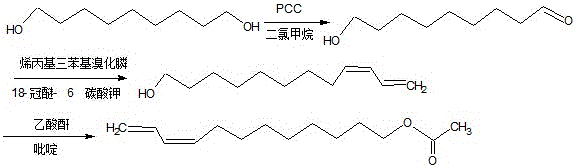
The synthetic method of z9,11-dodecadienol acetate
InactiveCN105061205BImprove protectionGood dispersionOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsSilurodiscoides
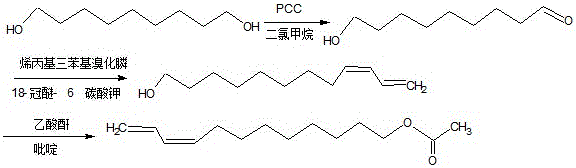
The invention discloses a synthesis method of Z9,11-dodecadienol acetate. The synthesis method comprises the following steps: (1) by taking 1,9-nonanediol as a raw material, performing unilateral oxidation to synthesize 9-hydroxyl-1-nonanal; (2) synthesizing an intermediate Z9,11-dodecadienol; (3) synthesizing the Z9,11-dodecadienol acetate. The synthesis method has the advantages of mild synthesis conditions, short reaction time, easy operation, wide application range, high yield, low cost and easy industrialization, the process flow is shortened, and three wastes are few. In a synthesis reaction of the 9-hydroxyl-1-nonanal, silica gel is added, the dispersion of PCC (Pyridinium Chlorochromate) is facilitated, the opportunity of oxidation of two hydroxyls is reduced by adding the PCC in batches, the reaction is performed at room temperature, and energy is saved; after the reaction is completed, ethyl ether and water are added for extraction, and the yield of reactants is improved. In the synthesis process of the Z9,11-dodecadienol, a phase transfer catalyst 18-crown ether-6 and non-polar solvent benzene are adopted to react so as to obtain a cis-product; meanwhile, weak alkali potassium carbonate is adopted, so that the protection of the hydroxyls of the 9-hydroxyl-1-nonanal is avoided, and a reaction synthesis route is shortened.
Owner:SHANXI AGRI UNIV
Synthesis method of 1H-imidazole-4-carboxylic acid
The invention discloses a synthesis method of 1H-imidazole-4-carboxylic acid. The method comprises steps as follows: (1) a hydrochloric acid solution is added to a reaction kettle, chromium trioxide is added under the stirring condition, the mixture is stirred uniformly and heated to 40-50 DEG C, pyridine is dropwise added, the mixture is stirred to react for 2-3 h after pyridine is dropwise added, PCC (pyridinium chlorochromate) is prepared, TiO2 is added, a dichloromethane solution of 4-methylimidazole is dropwise added under the stirring condition, the mixture is subjected to a reaction at40-50 DEG C for 3-4 h, then, filtration and centrifugation are performed, an organic layer is collected, reduced pressure distillation is performed, and 1H-imidazole-4-formaldehyde is prepared; (2) prepared 1H-imidazole-4-formaldehyde and dichloromethane are mixed, the obtained mixture is heated to 40-50 DEG C, KMnO4 is added, the mixture is mixed uniformly and stirred for a reaction for 3-4 h, filtration, reduced pressure distillation and recrystallization are performed, and 1H-imidazole-4-carboxylic acid is prepared. The synthesis method is simple to operate and mild in condition, fewer by-products are produced, the product purity is high and the product yield is higher.
Owner:ITIC MEDCHEM CO LTD
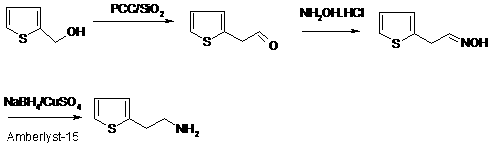
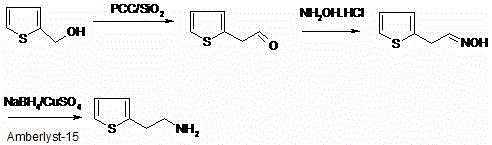
Synthetic method of 2-thiophene ethylamine
InactiveCN103288795AEasy to recycleReduce pollutionOrganic chemistryHydroxylamineHydroxylamine Hydrochloride
The invention discloses a synthetic method of 2-thiophene ethylamine. The method comprises the steps of oxidizing 2-thiophene ethanol to obtain 2-thiophene aldehyde by loaded pyridinium chlorochromate (PCC / SiO2), enabling the 2-thiophene aldehyde to react with hydroxylamine hydrochloride to obtain 2-thiophene acetaldehyde oxime; and reducing the 2-thiophene acetaldehyde oxime to obtain the 2-thiophene ethylamine through an NaBH4 / CuSO4 / Amberlyst-15 system. The method disclosed by the invention is mild in reaction condition, high in yield, small in pollution and suitable for industrialized production.
Owner:TAIYUAN UNIV OF TECH
Synthesis method of spirooxindole compounds
ActiveCN108440549AGreen and efficientAtom-free economyOrganic chemistryBulk chemical productionArylOrganic solvent
The invention relates to a synthesis method of spirooxindole compounds. The synthesis method comprises the steps as follows: spirooxindole compounds shown in a formula (I) are subjected to a rearrangement reaction in an organic solvent under the oxidation action of PCC (pyridinium chlorochromate), and spirooxindole compounds shown in a formula (II) are obtained, wherein the reaction temperature is20-120 DEG C, the reaction formula is shown in the description, Ar represents aryl or substituted aryl, and PG represents a protection group. The spirooxindole compounds are obtained through the one-step PCC oxidative rearrangement reaction, and the method has the advantages of simplicity and safety in operation, atom economy and capability of constructing multiple chemical bonds in one step.
Owner:INNOVATIVE DRUG RES & DEV CENT
Synthesis method of Z9,11-dodecadienol acetate
InactiveCN105061205AImprove protectionGood dispersionOrganic compound preparationCarboxylic acid esters preparationPtru catalystSilica gel
The invention discloses a synthesis method of Z9,11-dodecadienol acetate. The synthesis method comprises the following steps: (1) by taking 1,9-nonanediol as a raw material, performing unilateral oxidation to synthesize 9-hydroxyl-1-nonanal; (2) synthesizing an intermediate Z9,11-dodecadienol; (3) synthesizing the Z9,11-dodecadienol acetate. The synthesis method has the advantages of mild synthesis conditions, short reaction time, easy operation, wide application range, high yield, low cost and easy industrialization, the process flow is shortened, and three wastes are few. In a synthesis reaction of the 9-hydroxyl-1-nonanal, silica gel is added, the dispersion of PCC (Pyridinium Chlorochromate) is facilitated, the opportunity of oxidation of two hydroxyls is reduced by adding the PCC in batches, the reaction is performed at room temperature, and energy is saved; after the reaction is completed, ethyl ether and water are added for extraction, and the yield of reactants is improved. In the synthesis process of the Z9,11-dodecadienol, a phase transfer catalyst 18-crown ether-6 and non-polar solvent benzene are adopted to react so as to obtain a cis-product; meanwhile, weak alkali potassium carbonate is adopted, so that the protection of the hydroxyls of the 9-hydroxyl-1-nonanal is avoided, and a reaction synthesis route is shortened.
Owner:SHANXI AGRI UNIV
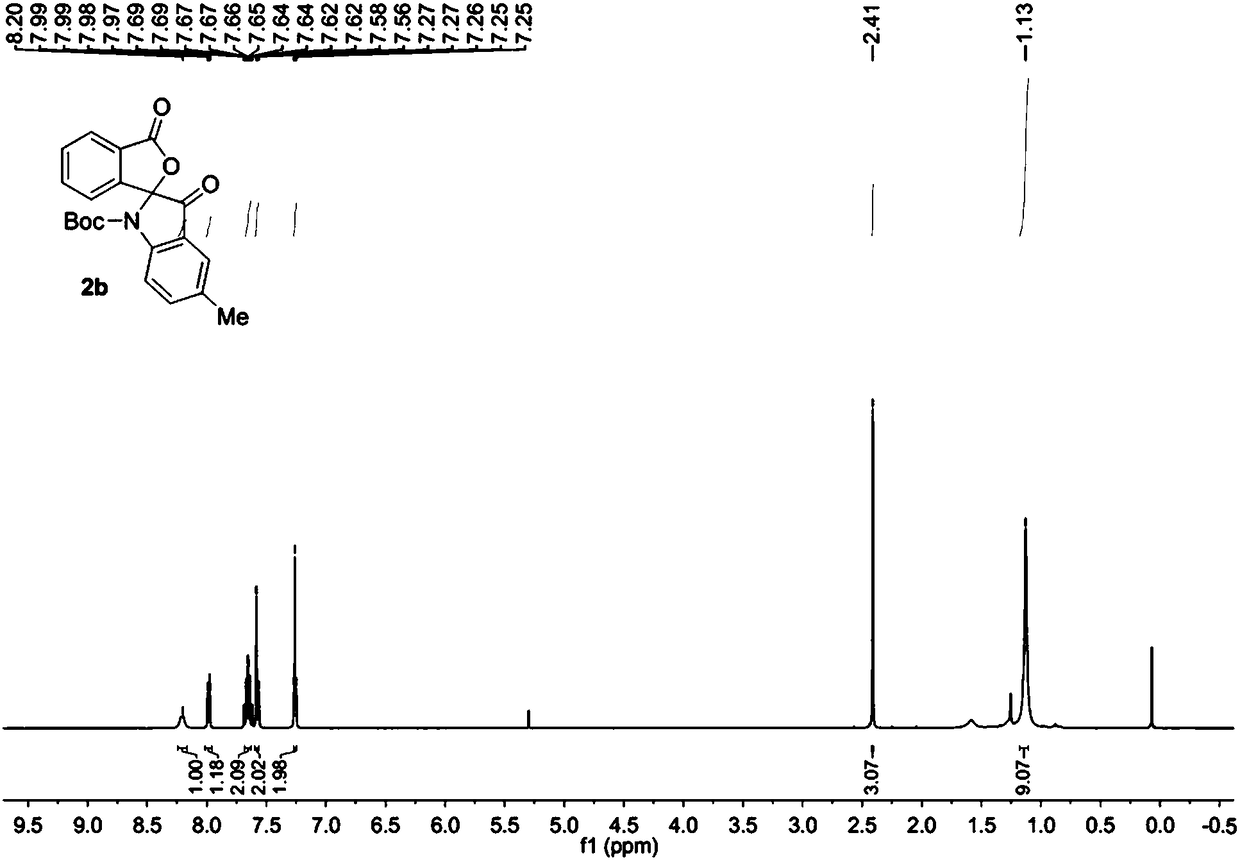
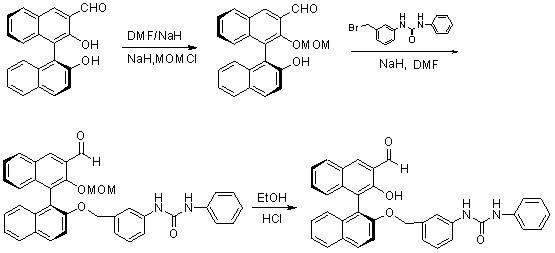
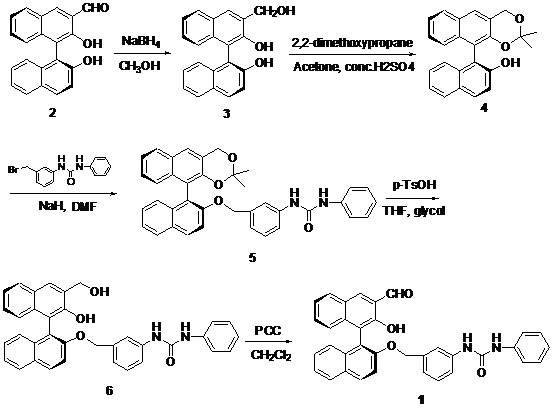
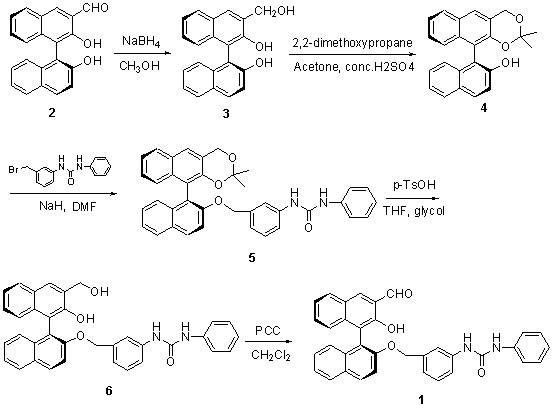
Method for synthesizing (S)-2-hydroxy-2'-(3-phenylureaphenyl)-1,1'-binaphthyl-3-formaldehyde
InactiveCN102070492ARich sourcesEasy to getUrea derivatives preparationOrganic compound preparationTosylic acidPtru catalyst
The invention discloses a method for synthesizing (S)-2-hydroxy-2'-(3-phenylureaphenyl)-1,1'-binaphthyl-3-formaldehyde, which uses widely available raw materials, makes operation easy, has high yield and produces little pollution. The method comprises the following process steps: dissolving 3-formoxyl-1,1'-binaphthol serving as a raw material in methanol, cooling in an ice bath, adding sodium borohydride, and reducing to obtain 3-hydroxymethyl-1,1'-binaphthol; dissolving 3-hydroxymethyl-1,1'-binaphthol in acetone serving as a solvent, adding 2,2'-dimethoxypropane to perform a reaction in the presence of a catalyst, extracting with an organic solvent, and obtaining (S)-2-hydroxy-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene by column chromatograpic separation; dissolving (S)-2-hydroxy-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)-naphthalene in dry dimethyl formamide (DMF) serving as a solvent, adding sodium hydride under an ice bath condition, reacting for 0.5 and 1 hours, adding 3-(4-methoxyphenyl)-ureido-benzyl bromide, reacting and obtaining (S)-2-(3-phenylureidobenzoxy)-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)- naphthalene by column chromatograpic separation; dissolving the (S)-2-(3-phenylureidobenzoxy)-1-(2,2-dimethyl-1,3-dioxo-1,2,3,4-tetrahydroanthracene-9-)- naphthalene in glycol serving as a solvent, heating to remove a protective group under the action of p-toluenesulfonic acid and reacting to obtain (S)-2-hydroxy-3-hydroxymethyl-2'-(3-tetrahydroanthracene)-1,1'-binaphthyl; and finally, oxidizing the (S)-2-hydroxy-3-hydroxymethyl-2'-(3-tetrahydroanthracene)-1,1'-binaphthyl in dry dichloromethane by pyridinium chlorochromate, and after reaction, filtering the reaction product by kieselguhr, concentrating and performing column chromatograpic separation to obtain the target product.
Owner:BOHAI UNIV
Preparation method of chilo suppressalis sex pheromone cis-11-hexadecenal
InactiveCN110922311AEasy to operateMild reaction conditionsOrganic compound preparationOrganic chemistry methodsHexyneP-Toluenesulfonic acid
The invention discloses a preparation method of chilo suppressalis sex pheromone cis-11-hexadecenal. The preparation method comprises the steps of (1) allowing 10-bromine-1-decanol and dihydropyran react to generate 10-bromine-1-decanol tetrahydropyran in a dichloromethane solution; (2) allowing the 10-bromine-1-decanol tetrahydropyrane obtained in the step (1) and 1-hexyne and n-butyllithium to react in a tetrahydrofuran solvent under the protection of nitrogen to generate 11-hexadecene tetrahydropyrane, and reacting the 11-hexadecene tetrahydropyrane with 1-hexyne and n-butyllithium to generate 11-hexadecene tetrahydropyrane; (3) carrying out a reduction reaction on the 11-hexadecene tetrahydropyran obtained in the step (2) and nickel chloride and sodium borohydride to generate cis-11-hexadecene tetrahydropyran in a methanol solution; (4) in methanol, enabling the cis-11-hexadecene tetrahydropyran obtained in the step (3) to react with p-toluenesulfonic acid to generate cis-11-hexadecenol; and (5) in dichloromethane, carrying out an oxidation reaction on the cis-11-hexadecenol obtained in the step (4) and pyridinium chlorochromate to generate the cis-11-hexadecenal. The method issimple to operate and mild in reaction condition. Medicines and reagents used in the reaction are conventional products, are low in price and are easy to obtain, and the reaction cost is reduced.
Owner:JILIN INST OF CHEM TECH
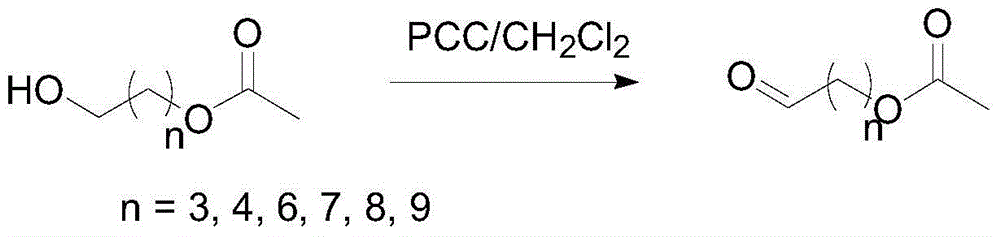
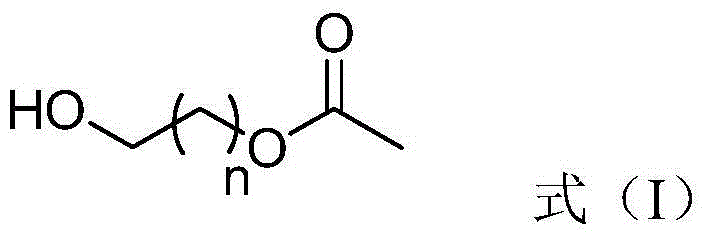
Preparation method for generating aldehyde by oxidizing primary alcohol having ester group
InactiveCN104557527AEasy post-processingOrganic compound preparationCarboxylic acid esters preparationSolventPrimary alcohol
The invention discloses a preparation method for generating aldehyde by oxidizing primary alcohol having an ester group, wherein the method includes the steps of under a stirring condition, allowing the primary alcohol having the ester group and represented by the formula (I) to generate an oxidation reaction in the presence of a solvent and under the action of an oxidant to generate the aldehyde; the oxidant contains an Al2O3 carrier and pyridinium chlorochromate loaded on the Al2O3 carrier, and with the oxidant total weight as a base, the content of the Al2O3 carrier is 1-99 wt%, and the content of pyridinium chlorochromate is 1-99 wt%. With adopting of the preparation method, oxidation can be completed in a shorter time, post-treatment is simpler and more convenient, and the defects that a product is bonded with chromium wastes and the like and is not easy to separate during single use of PCC are avoided.
Owner:CHINA PETROLEUM & CHEM CORP +1
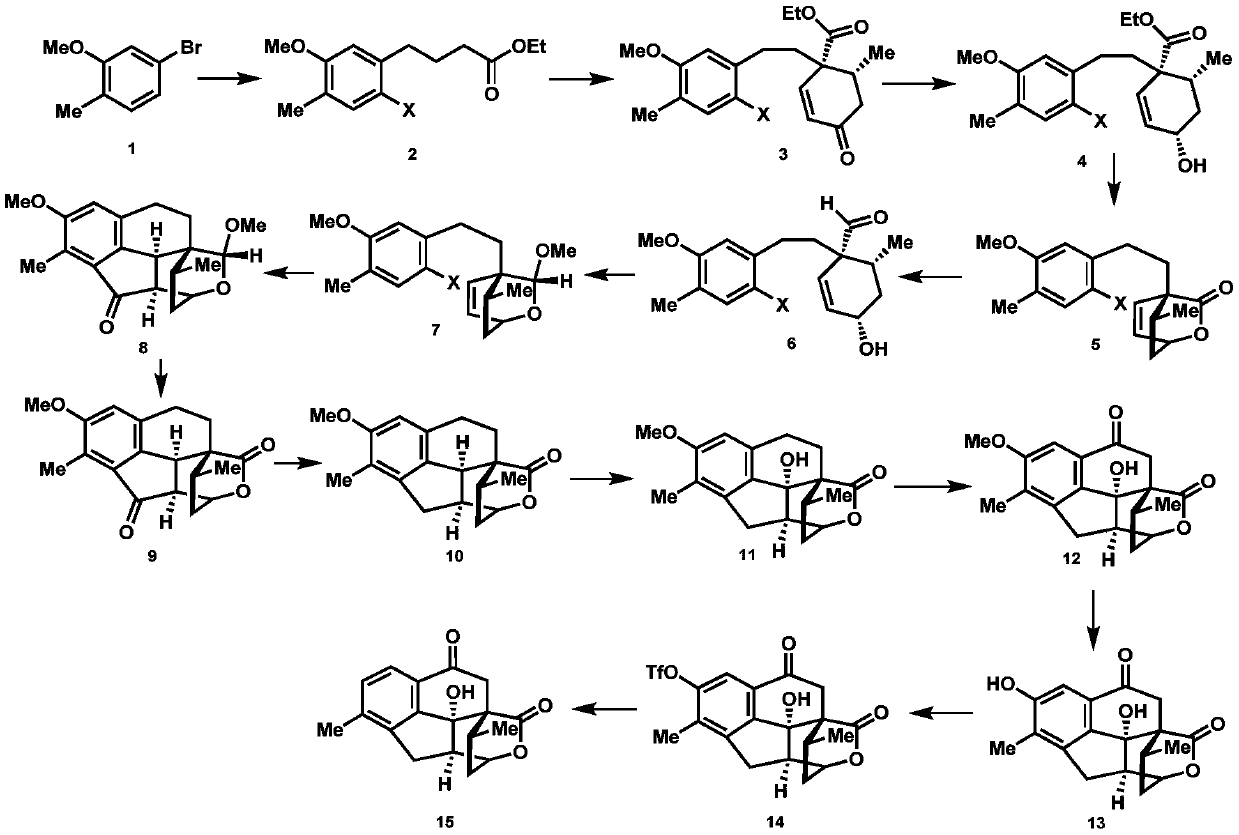
A kind of synthetic method of cephanolide C
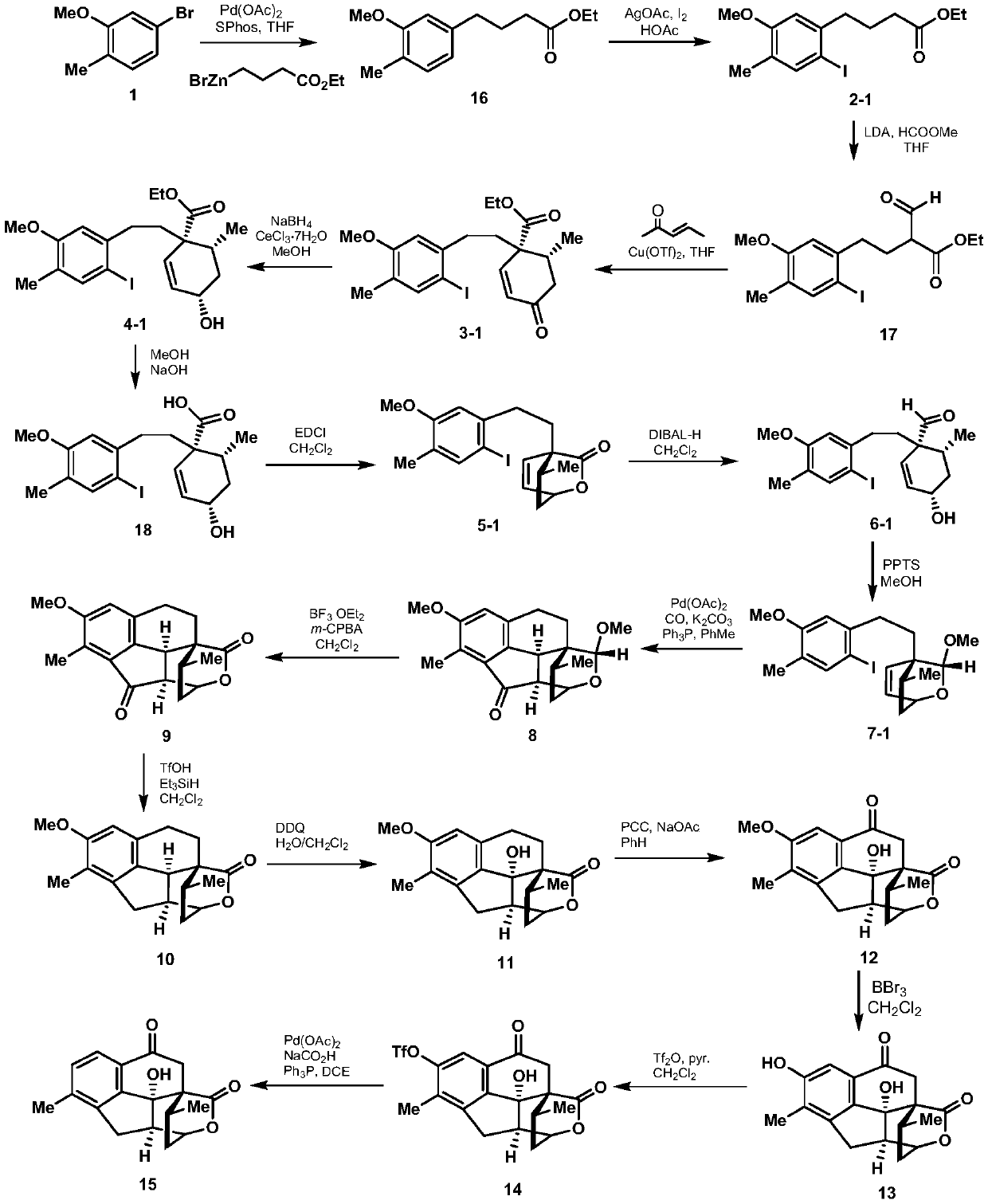
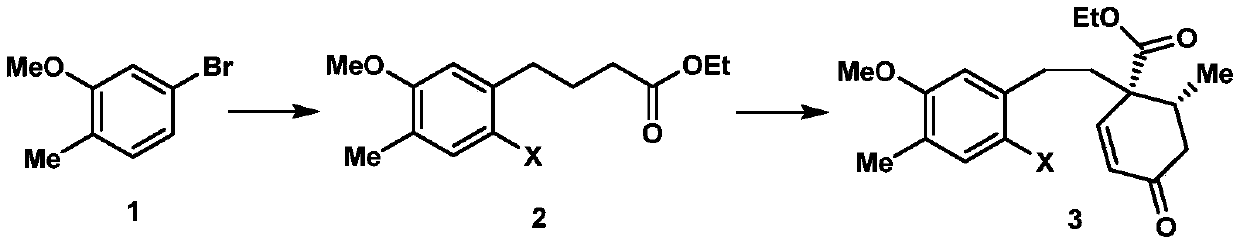
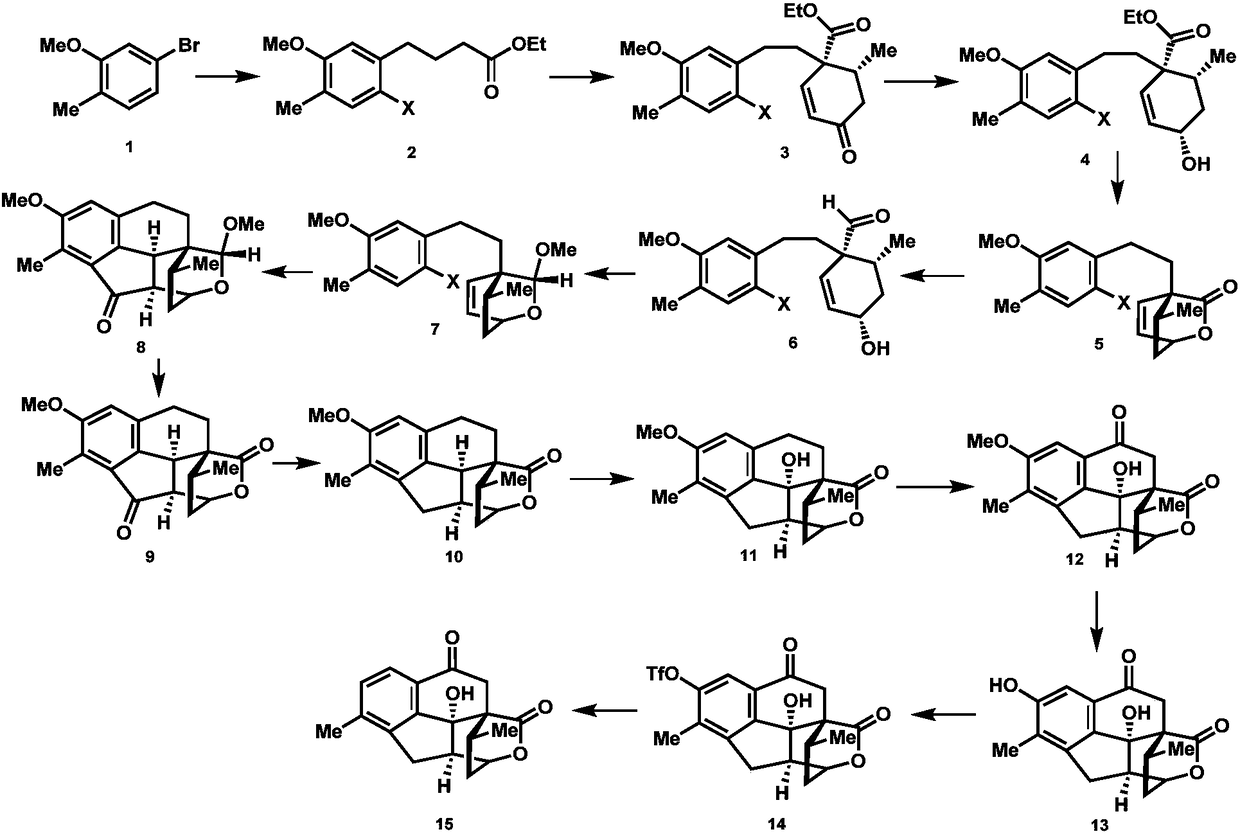
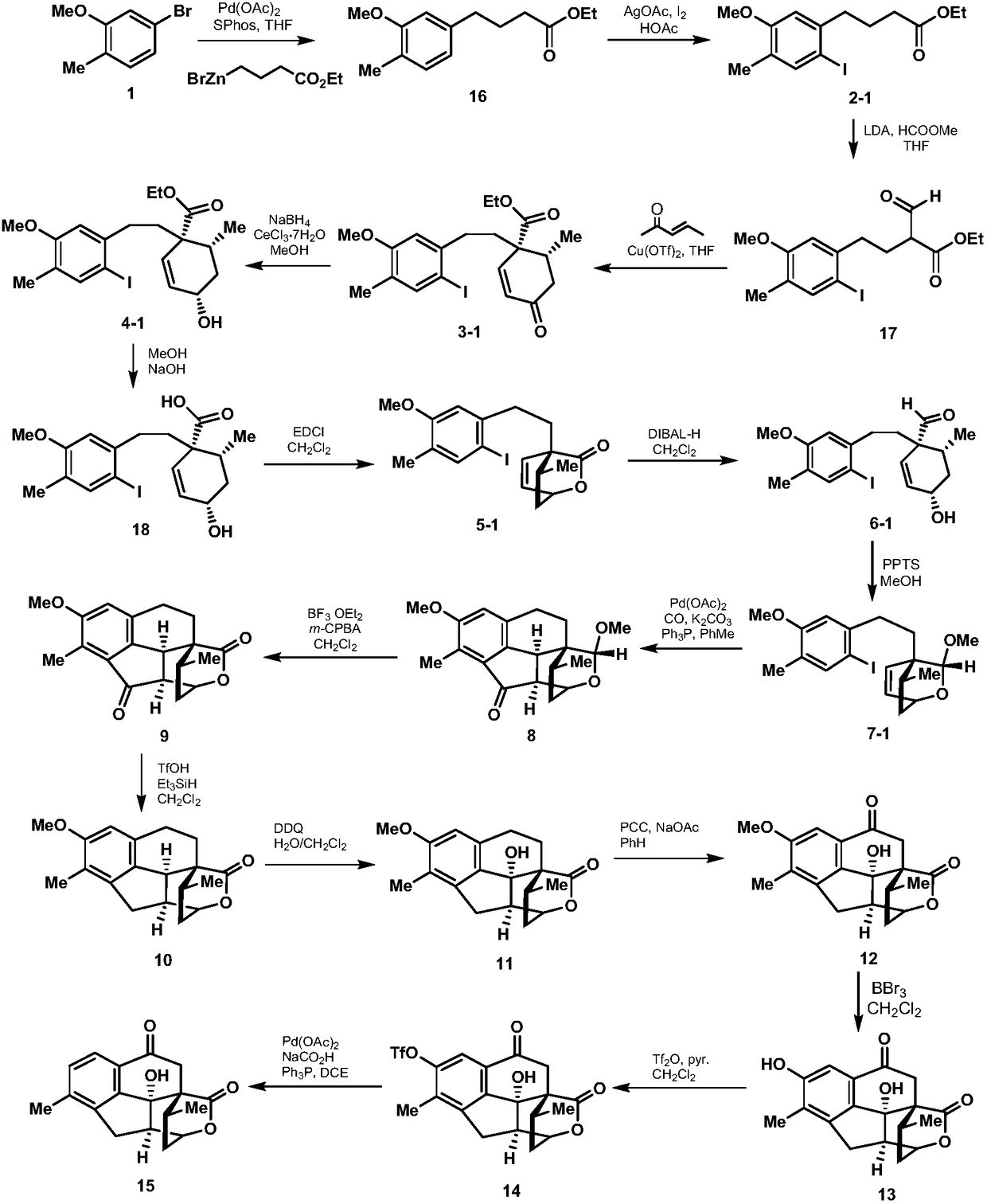
ActiveCN108129432BRealized the first chemical synthesisSimple and fast operationOrganic chemistryChemical synthesisFormylation reaction
Owner:SHAANXI NORMAL UNIV
Synthesis method of cephanolide C
ActiveCN108129432ARealized the first chemical synthesisSimple and fast operationOrganic chemistryChemical synthesisSynthesis methods
The invention discloses a synthesis method of cephanolide C. The method comprises the steps of taking commercially available 5-bromine-2-methylanisole as a synthesis raw material, performing Ei-ichi Negishi reaction, methoxy contraposition halogenation, and lithium diisopropylamide formylation reaction, allowing a product and 3-pentene-2-ketone to give Robinson cyclization reaction, performing Luche reduction, hydrolysis and esterification reaction to form a lactone compound, performing acid treatment after diisobutyl aluminum hydride reduction to form a Heck reaction precursor, performing palladium catalyzed carbonyl esterification coupling reaction, esterification and carbonyl reduction, then performing sequential oxidation, eemethylation and hydroxyl protection by trifluoromethylsulfonyl via 2,3-dichloro-5,6-dicyan para-quinone and pyridinium chlorochromate, and performing palladium catalyzed removal to achieve chemical synthesis of cephanolide C for the first time. The method has the advantages of concise and efficient synthesis route, easiness and simplicity in operation, low cost and the like, is applicable to massive synthesis of cephanolide C and provides an important material basis for bioactivity evaluation of a natural product, namely cephanolide C.
Owner:SHAANXI NORMAL UNIV
Synthesis process of 10-HDA (10-hydroxy-2-decenoic acid)
InactiveCN102603517AHigh yieldIncrease profitOrganic compound preparationCarboxylic compound preparationDecenoic AcidPtru catalyst
The invention relates to a new synthesis process of 10-HDA (10-hydroxy-2-decenoic acid) which comprises the following steps: preparing 8-bromo-1-octanol from 1,8-octanediol and hydrobromic acid under an action of a catalyst with toluene as a solvent; oxidizing 8-bromo-1-octanol with pyridinium chlorochromate in dichloromethane to obtain 8-bromooctanal; reacting obtained 8-bromooctanal with a phosphorus ylides reagent to obtain (2E)-10-bromo-2-decenoic acid; hydrolyzing obtained (2E)-10-bromo-2-decenoic acid in an alkali condition and then acidifying to obtain 10-HDA. The invention provides an industrial synthesis process of 10-HDA, which increases the utilization of atoms, has high purity and yield of 10-HAD and is simple and suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Synthetic method of 2-thiophene ethylamine
InactiveCN103288795BEasy to recycleReduce pollutionOrganic chemistryHydroxylamineHydroxylamine Hydrochloride
The invention discloses a synthetic method of 2-thiophene ethylamine. The method comprises the steps of oxidizing 2-thiophene ethanol to obtain 2-thiophene aldehyde by loaded pyridinium chlorochromate (PCC / SiO2), enabling the 2-thiophene aldehyde to react with hydroxylamine hydrochloride to obtain 2-thiophene acetaldehyde oxime; and reducing the 2-thiophene acetaldehyde oxime to obtain the 2-thiophene ethylamine through an NaBH4 / CuSO4 / Amberlyst-15 system. The method disclosed by the invention is mild in reaction condition, high in yield, small in pollution and suitable for industrialized production.
Owner:TAIYUAN UNIV OF TECH
3,6-dihydroxyl-22(27)imino-4-furan sterene and preparation method and application thereof
ActiveCN102643326BHigh anticancer activityLow toxicityOrganic active ingredientsDigestive systemFuranCancer cell
The invention provides a compound obtained by shifting 5,6-double bond of solasodine and carrying out functional group conversion, namely 3,6-dihydroxyl-22(27)imino-4-furan sterene. The compound has excellent action of resisting cancer cell growth, is high in activity, and can be used as a raw material for preparing anticancer medicaments for resisting leukaemia, colon cancer, rectum cancer, lung cancer and the like. The invention also provides a preparation method of the compound. The preparation method comprises the step of: based on 3 beta,16 beta-diacetoxy-26-chloro-5-cholestene-22-ketone as a raw material, carrying out 3-acetyl alcoholysis, pyridinium chlorochromate carrier reagent oxidation, reduction, amine cyclization and ring-closing reaction.
Owner:广东固升医药科技有限公司
Synthetic method of ridecevir D-ribonolactone
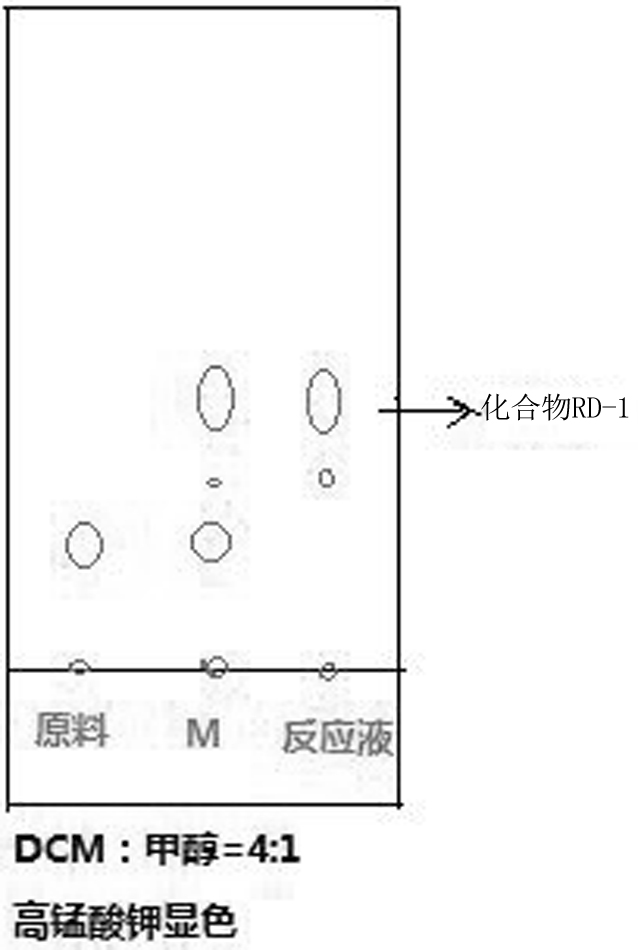
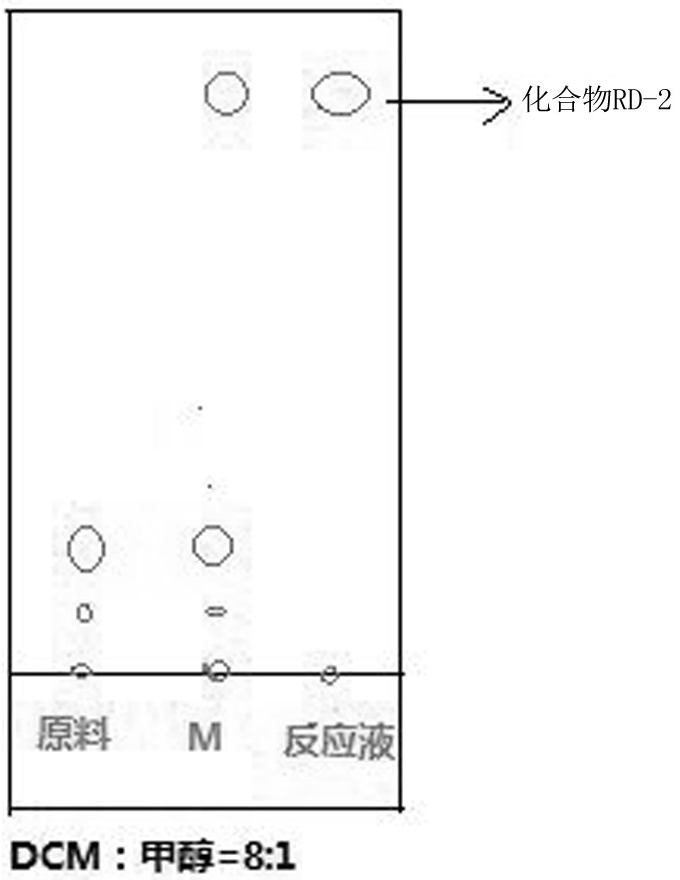
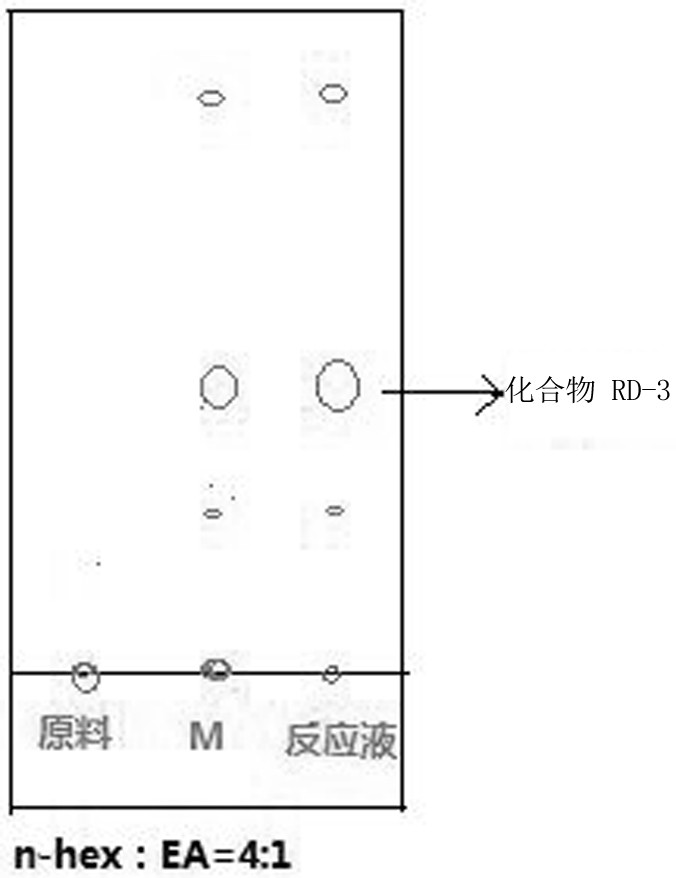
PendingCN114149446AAvoid it happening againShort synthetic routeOrganic chemistryBiochemical engineeringChemical compound
The invention discloses a synthesis method of ridecevir D-ribonolactone, which is characterized by comprising the following three synthesis steps: S1, preparation of a compound RD-1; s2, preparing a compound RD-2; and S3, preparing a target product compound RD-3. According to the method for synthesizing the ridecevir D-ribonolactone by using the cheap and easily available D-ribose as the starting material, the pyridinium chlorochromate oxidation reaction, the acetyl protection reaction, the acetonylidene protection hydroxyl reaction and other reactions are utilized, so that the synthesis process route is short, the operation difficulty is effectively simplified, the high-temperature reaction is avoided, and the method is suitable for industrial production. The synthesis process is safer and more reliable, the energy is effectively saved, the yield is improved, the synthesis process can be completed without using highly toxic substances, the generation of toxic and harmful wastewater is effectively avoided, and the environment is improved, so that the industrial production can be well realized.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
A kind of preparation method of ursodeoxycholic acid
The invention belongs to the field of medicine, and particularly relates to a method for preparing an ursodeoxycholic acid. The method comprises the following steps: performing esterification reaction to obtain a compound shown in formula II by taking an HDCA (hyodeoxycholic acid) as a starting raw material; performing selective hydroxy protection reaction to obtain a compound shown in formula III; performing hydroxy protection reaction to obtain a compound shown in formula IV; performing elimination reaction to obtain a compound shown in formula V; performing oxidation reaction on the compound shown in formula V under the action of tert-butyl hydroperoxide and pyridinium chlorochromate to obtain a compound shown in formula VI; performing hydrolysis to obtain a compound shown in formula VII; performing reduction to obtain the ursodeoxycholic acid shown in formula I. According to the method, the ursodeoxycholic acid is prepared by taking the HDCA as the starting raw material, so that not only can the problem of raw material shortage be solved, but also the method is convenient to operate, low in byproduct rate and cost, mild in reaction condition and suitable for large-scale production of the ursodeoxycholic acid.
Owner:青州市欣泰生物制品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com