Synthetic method of ridecevir D-ribonolactone
A synthesis method and ribonucleic acid technology are applied in the field of synthesis of Remdesivir D-ribonolactone, which can solve the problems of high environmental protection pressure, easy generation of impurities, numerous processes, etc., and achieve environmental protection pressure improvement, production cost reduction, The effect of simplifying the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
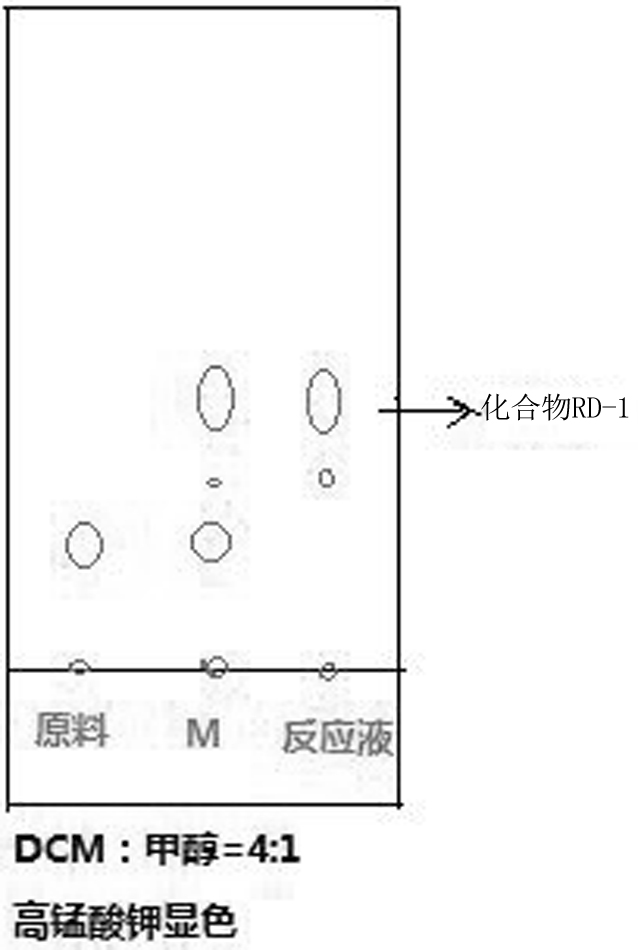
[0031] S1, preparation of compound RD-1
[0032] Weigh 100g, 0.67mol of D-ribose, 25.5g, 0.165mol, 0.25eq of diazabicyclo, 500ml of tetrahydrofuran, 158g, 0.73mol, 1.1eq of pyridinium chlorochromate;
[0033] First, fully stir and dissolve D-ribose, diazabicyclo and tetrahydrofuran according to the above-mentioned weighing amount, and then cool down to 0-5°C under the protection of nitrogen, and then slowly add pyridinium chlorochromate in batches. Reactions were carried out for 2 to 3 hours under monitoring (e.g. figure 1 shown), after the reaction is completed, add 6.5g, 62.5mmol of sodium bisulfite to quench at 0-5°C, stir for 30 minutes, then concentrate at 45-50°C under vacuum to form a reaction solution. Then hot filtration was carried out with absolute ethanol, and concentrated to obtain 85 g of compound RD-1 as a white solid, with a yield of 86%.
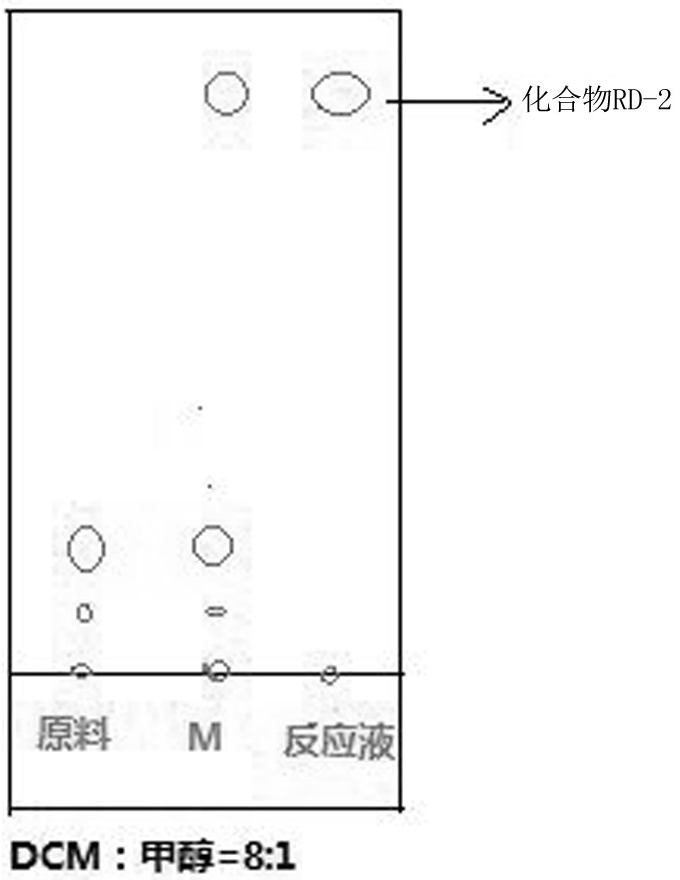
[0034] S2, preparation of compound RD-2
[0035] Weigh 70g, 0.5mol of compound RD-1, 350g of dichloromethane, 0.6g, 0.0...
Embodiment 2
[0041] S1, preparation of compound RD-1
[0042]Weigh 100g, 0.67mol of D-ribose, 20.5g, 0.135mol, 0.20eq of diazabicyclo, 500ml of tetrahydrofuran, 288g, 1.34mol, 2.0eq of pyridinium chlorochromate;
[0043] First, stir D-ribose with diazabicyclo and tetrahydrofuran according to the above weighing amount, and then cool down to 0-5°C under the protection of nitrogen, then slowly add pyridinium chlorochromate in batches, and carry out under TLC monitoring. 2 to 3 hours of reaction (such as figure 1 shown), after the reaction is completed, add 6.5g, 62.5mmol of sodium bisulfite to quench at 0-5°C, stir for 30 minutes, concentrate under vacuum at 45-50°C to form a reaction solution, and then Filtrated with absolute ethanol and concentrated to obtain 66 g of compound RD-1 as a white solid, with a yield of 65%.
[0044] S2, preparation of compound RD-2
[0045] Weigh 60g, 0.40mol of compound RD-1, 330g of dichloromethane, 0.5g, 0.004mol, 0.01eq of 4-dimethylaminopyridine, 41.4g, ...
Embodiment 3
[0051] S1, preparation of compound RD-1
[0052] Weigh 100g, 0.67mol of D-ribose, 25.5g, 0.165mol, 0.25eq of diazabicyclo, 500ml of 50% tetrahydrofuran aqueous solution, 100g, 0.5mol, 0.6eq of pyridinium chlorochromate;
[0053] First, fully stir and dissolve D-ribose, diazabicyclo and 50% tetrahydrofuran aqueous solution according to the above weighing amount, and then cool down to 0-5°C under the protection of nitrogen, and then slowly add pyridinium chlorochromate in batches , and reacted for 2 to 3 hours under TLC monitoring (such as figure 1 shown), after the reaction is completed, add 6.5g, 62.5mmol of sodium bisulfite to quench at 0-5°C, stir for 30 minutes, concentrate at 45-50°C under vacuum to form a reaction solution, and then After hot filtration with absolute ethanol, 60 g of white solid compound RD-1 was obtained by concentration, and the yield was 59%.
[0054] S2, preparation of compound RD-2
[0055] Weigh 60g, 0.40mol of compound RD-1, 300g of dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com