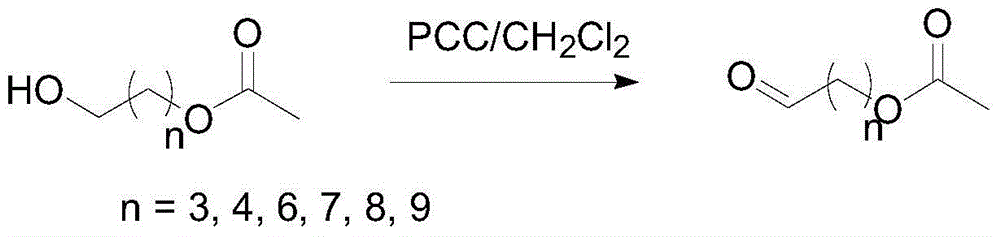
Preparation method for generating aldehyde by oxidizing primary alcohol having ester group
A technology of ester group and primary alcohol, which is applied in the field of preparation of aldehyde group by oxidation of alcohol hydroxyl group, can solve the problems of long oxidation time, difficult filtration and separation, etc., and achieves the effect of simple and convenient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
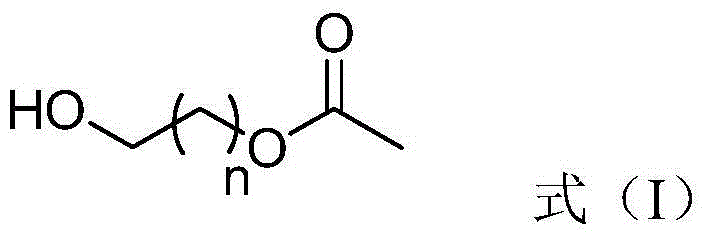
[0020] The invention provides a method for preparing a corresponding aldehyde from a primary alcohol with an ester group. The preparation method may include: under stirring conditions, the primary alcohol with an ester group represented by the formula (I) in the presence of a solvent Oxidation reaction occurs under the action of oxidizing agent to generate aldehyde; said oxidizing agent contains Al 2 o 3 Carriers and loads in Al 2 o 3 Pyridinium chlorochromate on the carrier, and based on the total weight of the oxidizing agent, the Al 2 o 3 The content of the carrier can be 1-99% by weight, and the content of the pyridinium chlorochromate can be 1-99% by weight,
[0021]
[0022] Wherein, n=2, 3, 4, 5, 6, 7, 8 or 9; preferably, n=2, 5, 8 or 9.
[0023] According to the present invention, based on the total weight of the oxidizing agent, the Al 2 o 3 The content of the carrier may be 1-99% by weight, and the content of the pyridinium chlorochromate (PCC) may be 1-99%...
Embodiment 1
[0036] Step 1: In a 50mL beaker, dissolve 4.2g PCC in 8mL distilled water, heat to 40 degrees Celsius and stir until PCC is completely dissolved, add 13.0g Al 2 o 3 The powder was stirred evenly at a stirring rate of 700 rpm; the solid was transferred to a watch glass, and dried in vacuum overnight to obtain an orange solid for later use.
[0037] Step 2: In a 100mL round-bottomed flask, dissolve 1.0mmol of primary alcohol with an ester group (wherein, in structural formula (I), n=2) in 50mL of dichloromethane, and slowly add 2.5g of PCC in batches at room temperature / Al 2 o 3 Oxidant powder, stirred vigorously for 25 minutes. After the completion of the reaction as monitored by GC, the solid was filtered off, and the filtrate was concentrated to obtain the product aldehyde with an ester group, with a yield of 97.2%.
Embodiment 2
[0039] The first step: the same as the first step in embodiment 1.
[0040] Step 2: In a 100mL round-bottomed flask, dissolve 1.0mmol of primary alcohol with an ester group (n=5) in 50mL of dichloromethane, and slowly add 2.5g of PCC / Al in batches at room temperature 2 o3 Oxidant powder, stirred vigorously for 30 minutes. After the completion of the reaction as monitored by GC, the solid was filtered off, and the filtrate was concentrated to obtain the product aldehyde with an ester group, with a yield of 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com