Method for synthesis of sex pheromone compound of grapholitha molesta
The invention relates to a technology for a sex pheromone and a synthetic method of the pear worm, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., and can solve the problems such as difficulty in obtaining raw materials, harsh reaction conditions, and excessively long routes, so as to shorten the production time. Period, mild reaction conditions, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
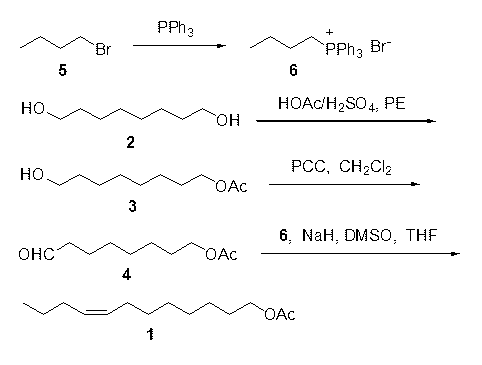
[0017] Embodiment 1 8-hydroxyl undecyl alcohol acetate ( 3 ) Preparation: 1,8-octanediol ( 2 ) (14.6 g, 0.1 mol) dissolved in 450ml petroleum ether, add a catalytic amount of 98% concentrated sulfuric acid, slowly add 50ml petroleum ether solution of glacial acetic acid (6 g, 0.1 mol) dropwise, continue to cool at 60-90℃ Stop the reaction after reflux reaction for 10 hours, add saturated sodium carbonate to neutralize to pH = 7-8, separate the toluene layer, extract the water layer with toluene 3 times, combine the organic layers, dry over anhydrous sodium sulfate and evaporate under reduced pressure After solvent, the crude product was obtained, and purified by column chromatography (eluent was sherwood oil and ethyl acetate) to obtain 8-hydroxyoctyl alcohol acetate ( 3 ) 15.4 g, yield 82%.
Embodiment 2
[0018] Embodiment 2 8-oxooctanyl acetate ( 4 ) preparation: 8-hydroxy octanol acetate ( 3 ) (15 g, 80 mmol) was dissolved in 400 ml of dry dichloromethane, cooling in an ice bath and adding pyridinium chlorochromate (25.86 g, 120 mmol) in batches under stirring, stirring at room temperature for 3 hours, adding an appropriate amount of water, separated dichloromethane, and extracted 2 times with dichloromethane, combined the organic layers, dried over anhydrous sodium sulfate and evaporated the solvent to obtain a crude product, which was purified by column chromatography (eluent was petroleum ether and ethyl acetate ) was purified to obtain 8-oxooctanyl acetate ( 4 ) 11.3 g, yield 75%, the crude product can also be directly used in the next reaction.
Embodiment 3
[0019] Embodiment 3 n-butyltriphenylphosphine bromide ( 6 ) preparation: n-bromobutane ( 5 ) (0.1 mol, 13.7 g) and triphenylphosphine (0.1 mol, 26.2 g) were heated to 90°C for 30 hours, washed twice with ether to obtain n-butyltriphenylphosphine bromide ( 6 )38 g, yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com