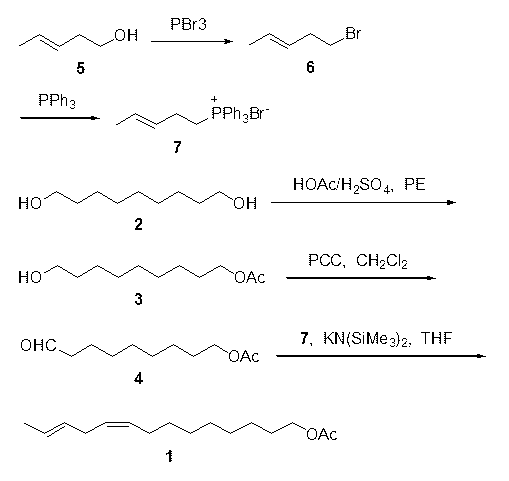
Synthesis of plodia interpunctella sex pheromone 9Z, 12E-tetradecadiene-1-acetate
A technology of alcohol acetate and pheromone, which is applied in the field of synthesis of sex pheromone 9Z, 12E-tetradecadien-1-ol acetate of Indian meal moth, can solve problems such as limitation of control methods, and achieve reduction The effect of production cost, mild reaction conditions and shortened production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 9-hydroxy nonanol acetate ( 3 ) preparation: Dissolve 1,9-nonanediol (16 g, 0.1 mol) in 450 ml of petroleum ether, add a catalytic amount of 98% concentrated sulfuric acid, slowly add glacial acetic acid (6 g, 0.1 mol) in 50 ml of petroleum Ether solution, continue to reflux at 60-90°C for 18 hours after dripping, stop the reaction, add saturated sodium carbonate to neutralize to pH=7-8, separate the petroleum ether layer, extract the water layer with petroleum ether for 3 times, combine the organic layer, dried over anhydrous sodium sulfate and distilled off the solvent under reduced pressure to obtain a crude product, which was purified by column chromatography (petroleum ether and ethyl acetate as the eluent) to obtain 9-hydroxynonanol acetate ( 3 ) 16.3 g, yield 81%.
Embodiment 2
[0019] Embodiment 2 9-Oxononanol acetate ( 4 ) preparation: 9-hydroxynonanol acetate ( 3 ) (16.2 g, 80 mmol) was dissolved in 400 ml of dry dichloromethane, cooling in an ice bath and adding pyridinium chlorochromate (25.86 g, 120 mmol) in batches under stirring, stirring at room temperature for 3 hours, adding an appropriate amount of water, separated dichloromethane, and extracted 2 times with dichloromethane, combined the organic layers, dried over anhydrous sodium sulfate and evaporated the solvent to obtain a crude product, which was purified by column chromatography (eluent was petroleum ether and ethyl acetate ) was purified to obtain 9-oxononanol acetate ( 4 ) 12.3 g, yield 77%, the crude product can also be directly used in the next reaction.
Embodiment 3
[0020] Example 3 5-bromo-3E-pentene ( 6 ) preparation: Dissolve trans-3-penten-1-ol (0.15 mol, 12.9 g) in 150 ml of dry dichloromethane, add phosphorus tribromide (55 mmol, 14.9 g) dropwise, continue to After stirring in an ice bath for 3 hours, add saturated sodium carbonate to neutralize to pH = 7-8, separate the organic layer, extract the water layer once with dichloromethane, combine the organic layers, dry over anhydrous sodium sulfate and evaporate under reduced pressure. Obtain crude product after solvent removal, obtain 5-bromo-3E-pentene ( 6 ) 17.0 g, yield 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com