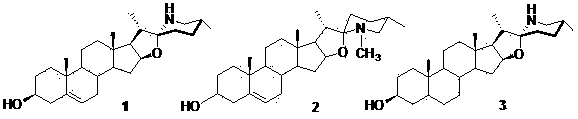
3,6-dihydroxyl-22(27)imino-4-furan sterene and preparation method and application thereof
A technology of furostene and dihydroxy, which is applied in the field of chemical biology to achieve good anticancer activity, low toxicity and teratogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Synthesis of compound 3,6-dihydroxy-22(27)imino-4-furostene :
[0030] (1) With 3β, 16β-diacetoxy-26-chloro-5-cholesten-22-one Ⅰ As raw material, using weak base K 2 CO 3 , the selective hydrolysis of 3-acetoxy in a mixture of tetrahydrofuran and methanol (see Cheng Shuilian. Master's Thesis, Yunnan University, Kunming, 2007, p. 20) to obtain 3β-hydroxy-16β-acetoxy- 26-Chloro-5-cholesten-22-one Ⅱ , yield 82%, melting point: 167.0-168.0 ℃;
[0031] (2) Silica-supported pyridinium chlorochromate (PCC) was prepared from concentrated hydrochloric acid, chromium trioxide and silica gel [see Meng Qingyong, Wu Manjiang·Aili, Zhang Lijing, etc. Applied Chemical Industry, 2008, 37(3): 314-316 ], 4.0 g (8 mmol) of compound Ⅱ Dissolve in 100 mL anhydrous CH 2 Cl 2 26 g (48 mmol) PCC carrier reagent was added in batches under stirring, and the reaction was stirred at room temperature for 25 hours. After the reaction was completed, the solvent was removed under reduced p...
Embodiment 2
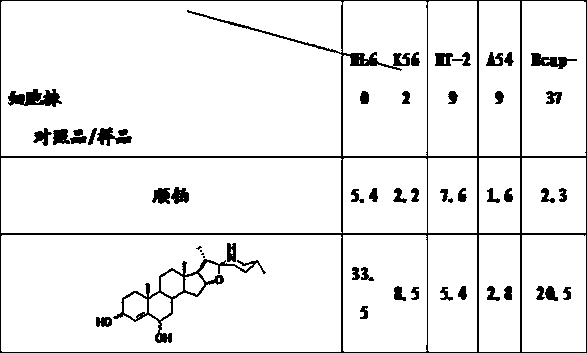
[0046] Inhibitory effect of 3,6-dihydroxy-22(27)-imino-4-furostene on the growth of tumor cells
[0047] The specific operation method of the activity test: the tumor lines used in the test were purchased from the Cell Resource Center of the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, and all the tumor lines were cultured in 1640 complete medium containing 10% fetal bovine serum (37°C, 5% CO 2 condition). During the test, the cells in the logarithmic growth phase were adjusted to an appropriate concentration (suspension cells 6×10 4 / mL, adherent cells 5×10 4 / mL) into a 96-well culture plate, 90 mL / well. Set 5 concentrations of the drug, and set 3 parallel wells for each concentration, 10 mL / well, so that the tested concentrations are 0.01, 0.1, 1, 10, 100 mg / mL; blank wells are 100 mL of culture solution, negative control wells Add 10 mL of culture medium to 90 mL of cells; the positive control is the anticancer drug cisplatin. The adherent gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com